The distillation column in the Michigan Tech Unit Operations Lab distills a mixture of ethanol and water.
Question:
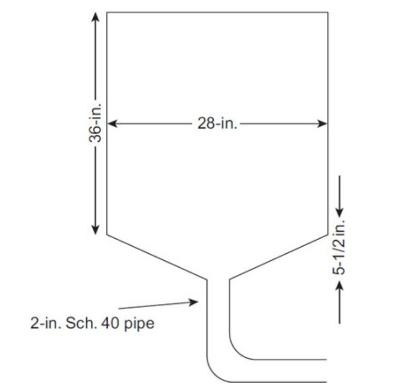
The distillation column in the Michigan Tech Unit Operations Lab distills a mixture of ethanol and water. The ethanol product is delivered to tank T-107 for temporary storage. The tank is a vertical cylinder in geometry with a cone-shaped bottom, as shown in Figure 4-19. A 2-in. schedule 40 pipe is connected to the bottom of the tank to pump the tank back to the raw material tank. We want to determine the consequences due to the rupture of the pipe at the very bottom of the vessel and spillage and evaporation of the ethanol.
Typically, during normal operation, tank T-107 is approximately \(20 \%\) full. However, for our purposes, we will assume a more conservative \(50 \%\) full. Also, the ethanol is about \(90 \%\) in purity, but we will consider the more conservative case of \(100 \%\) ethanol.
Figure 4-18
Supplemental information:
Ethanol: \(\mathrm{C}_{2} \mathrm{H}_{6} \mathrm{O}\)
Specific gravity of ethanol: 0.7893 2-in. schedule 40 pipe: \(\mathrm{ID}=2.067\) in., \(\mathrm{OD}=2.375\) in.
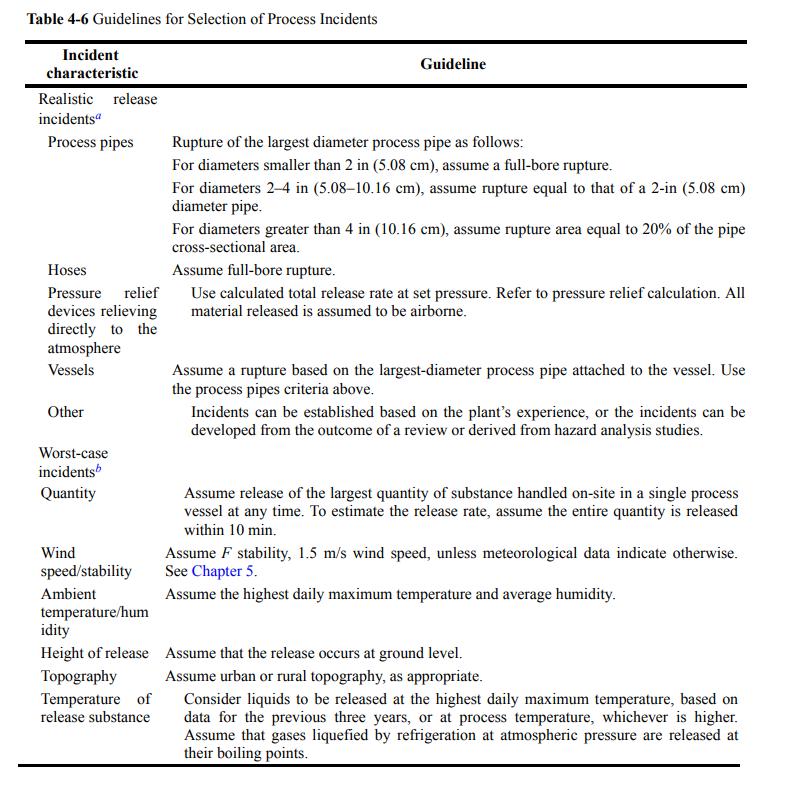
a. Use Table 4-6 to determine an appropriate pipe diameter for the realistic release case of the pipe rupture at the base of the tank. Should you use the ID or OD of the pipe?
b. What is the initial discharge rate when the pipe initially ruptures? The tank is padded with nitrogen at a regulated pressure of \(4 \mathrm{in}\). of water gauge.
c. If the initial discharge rate is maintained, how long will it take to drain all of the material from the tank? If the total draining time is less than \(10 \mathrm{~min}\), then the release can be considered instantaneous. Is this the case?
d. If an ethanol pool forms on the floor with an estimated liquid depth of \(1 \mathrm{~cm}\), what is the pool area, in \(\mathrm{ft}^{2}\) ?
e. Estimate the evaporation rate of the ethanol from the pool, in \(\mathrm{lb}_{\mathrm{m}} / \mathrm{s}\). Assume a temperature of \(80^{\circ} \mathrm{F}\) and an ambient pressure of \(1 \mathrm{~atm}\).
f. Estimate the concentration of ethanol vapors in the UO lab (in ppm) from this evaporation. Assume a UO lab volume of 74,300 \(\mathrm{ft}^{3}\) and a ventilation rate of 6 air changes per hour.
Step by Step Answer:

Chemical Process Safety Fundamentals With Applications
ISBN: 9780134857770
4th Edition
Authors: Daniel A. Crowl, Joseph F. Louvar





