Question: Using TLV data from Appendix E, convert TLV in ppm to mg/m3 for the following compounds. Assume a temperature of 25C and a pressure of
Using TLV data from Appendix E, convert TLV in ppm to mg/m3 for the following compounds.
Assume a temperature of 25°C and a pressure of 1 atm.
a. benzene
b. chlorine
c. cyclohexanol
d. ethylene oxide
Appendix E,
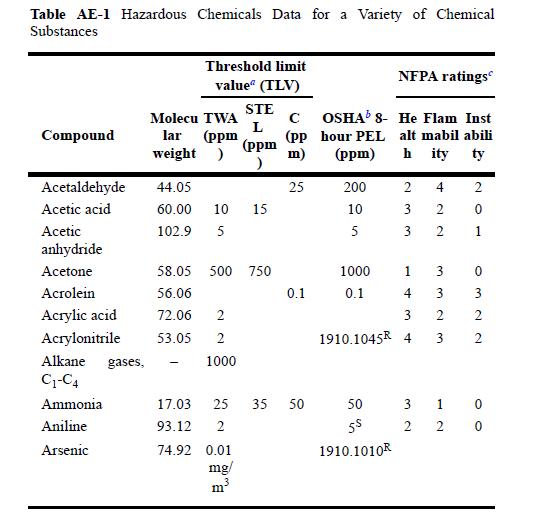
Table AE-1 Hazardous Chemicals Data for a Variety of Chemical Substances Compound Acetaldehyde Acetic acid Acetic anhydride Acetone Acrolein Acrylic acid Acrylonitrile Alkane gases, C-C4 Ammonia Aniline Arsenic Threshold limit value (TLV) Molecu TWA lar (ppm weight ) STE L (ppm 44.05 60.00 10 15 102.9 5 58.05 500 750 56.06 72.06 2 53.05 2 1000 74.92 0.01 mg/ m (pp m) 25 0.1 17.03 25 35 50 93.12 2 NFPA ratings OSHA 8- He Flam Inst hour PEL alt mabil abili (ppm) hity ty 200 10 5 1000 0.1 2 4 3 2 3 2 1 4 3 1910.1045 4 50 55 1910.1010R 3 3 2 3 3 1 2 2 2 0 H 0 322 0 0
Step by Step Solution
3.42 Rating (149 Votes )
There are 3 Steps involved in it
The ideal gas law equation must be applied in order to convert TLV values from ppm to mgm mgm Three ... View full answer

Get step-by-step solutions from verified subject matter experts


