Consider the following elementary gas-phase reversible reaction to be carried out isothermally with no pressure drop and
Question:
Consider the following elementary gas-phase reversible reaction to be carried out isothermally with no pressure drop and for an equal molar feed of A and B with CA0 = 2.0 mol/dm3. 2A + B ⇄ C
a. What is the concentration of B initially? CB0 = ____ (mol/dm3)
b. What is the limiting reactant? ______
c. What is the exit concentration of B when the conversion of A is 25%? CB = _____ (mol/dm3)
d. Write –rA solely as a function of conversion (i.e., evaluating all symbols) when the reaction is an elementary, reversible, gas-phase, isothermal reaction with no pressure drop with an equal molar feed and with CA0 = 2.0 mol/dm3, kA = 2 dm6/mol2•s, and KC = 0.5 all in proper units –rA = ____.
e. What is the equilibrium conversion?
f. What is the rate when the conversion is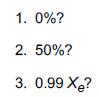
Step by Step Answer:






