Corrosion of high-nickel stainless steel plates was found to occur in a distillation column used at DuPont
Question:
Corrosion of high-nickel stainless steel plates was found to occur in a distillation column used at DuPont to separate HCN and water. Sulfuric acid is always added at the top of the column to prevent polymerization of HCN. Water collects at the bottom of the column and HCN collects at the top. The amount of corrosion on each tray is shown in Figure P3-8B as a function of plate location in the column.
Figure P3-8B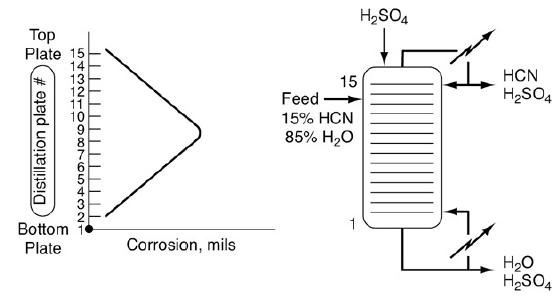
Corrosion in a distillation column. The first image shows the amount of corrosion in each tray as a function of plate location in the column. The distillation plate is numbered from bottom to top, ranging from 1 to 15. The corrosion is least at 1, gradually increases as the number increases. It is maximum at around 8 and 9, and then gradually decreases until 15. The second image shows the corrosion of high-nickel stainless steel plates in a distillation column used at DuPont to separate HCN and water. Arrows represent the following: Sulphuric acid is added at the top of the column. The feed of 15 percent HCN and 85 percent water is fed into the distillation column. It shows that the water
collects at the bottom of the column and HCN collects at the top.
The bottommost temperature of the column is approximately 125°C and the topmost is 100°C. The corrosion rate is a function of temperature and the concentration of an HCN–H2SO4 complex.
1. Suggest an explanation for the observed corrosion-plate profile in the column. What effect would the column operating conditions have on the corrosion-plate profile?
2. Identify the components of the GHS diagram that you could apply to this problem.
Step by Step Answer:






