Ethyl acetate is an extensively used solvent and can be formed by the vapor-phase esterification of acetic
Question:
Ethyl acetate is an extensively used solvent and can be formed by the vapor-phase esterification of acetic acid and ethanol.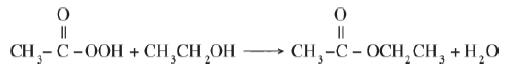
The chemical equation shows the formation of Ethyl acetate by the vapor phase esterification of acetic acid and ethanol. Methyl methanoate (CH3 single-bonded with C single-bonded with OOH and double-bonded with O) reacts with Ethanol (CH3CH2OH) which tends to form Butanone (CH3 single-bonded with C single-bonded with OCH2CH3, double-bonded with O and water (H2O).
An NFPA label for Acetic acid is shown. The blue diamond indicates a health hazard, the red diamond indicates a fire hazard, the white diamond indicates a specific hazard, and the red diamond indicates the reactivity. For Acetic acid, the health hazard is 3 which is of extreme danger, the fire hazard is 2, and the reactivity is 0 that is stable, and the specific hazard is left blank. The reaction was studied using a microporous resin as a catalyst in a packed-bed microreactor (Ind. Eng. Chem. Res., 26(2), 198(1987)). The reaction is first-order in
ethanol and pseudo zero-order in acetic acid. The total volumetric feed rate is 25 dm3/min, the initial pressure is 10 atm, the temperature is 223°C, and the pressuredrop parameter, α, equals 0.01 kg–1. For an equal molar feed rate of acetic acid and ethanol, the specific reaction rate is about 1.3 dm3/kg-cat-min.
a. Calculate the maximum weight of catalyst that one could use and maintain an exit pressure above 1 atm.
b. Write out the CRE algorithm and then solve these equations analytically to determine the catalyst weight necessary to achieve 90% conversion.
c. Write a Polymath program to plot and analyze X, p, and f = υ/υ0 as a function of catalyst weight down the packed-bed reactor. You can either use your analytical equations for x, p, and f or you can plot these quantities using the Polymath program.
d. What is the ratio of catalyst needed to achieve the last 5% (85%–90%) conversion to the weight necessary to achieve the first 5% conversion (0%–5%) in the reactor? Note: You can use the results in part (c) to also answer this part.
Step by Step Answer:






