Experimental data for the gas-phase catalytic reaction A + B C is shown below. The limiting
Question:
Experimental data for the gas-phase catalytic reaction A + B → C is shown below. The limiting step in the reaction is known to be irreversible, so that the overall reaction is irreversible. The reaction was carried out in a differential reactor to which A, B, and C were all fed. Run Number PA (atm) PB (atm) PC (atm) Reaction rate (mol)/(g-cat · s)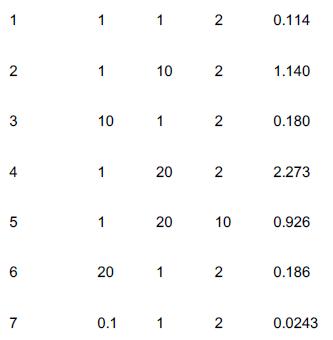
a. Suggest a rate law consistent with the experimental data.
b. From your rate expression, which species can you conclude are adsorbed on the surface?
c. Suggest a rate law and then show that your mechanism is constant with the rate law in part (a).
d. For an entering partial pressure of A of 2 atm in a PBR, what is the ratio of A to C sites at 80% conversion of A?
e. At what conversion are the number of A and C sites equal? (Ans: X = 0.235)
f. What reactor volume is necessary to achieve 90% conversion of A for a stoichiometric feed and flow of A 2 mol/s? (Ans: W = 8.9 g-cat)
If necessary, feel free to use none, any, or all of the following parameter values:
k=2.5molatm2g-cat⋅S,KA=4 atm−1,KC=13atm−1,KI=10 atm−1
Step by Step Answer:






