Question: Exploring the Example Problems. 1. Example 3-1. Activation Energy. In the Excel spreadsheet, replace the value of k at 312.5 K with k = 0.0009
Exploring the Example Problems.
1. Example 3-1. Activation Energy. In the Excel spreadsheet, replace the value of k at 312.5 K with k = 0.0009–1s and determine the new values of E and k.
2. Example 3-1. Make a plot of k versus T and ln k versus (1/T) for E = 240 kJ/mol and for E = 60 kJ/mol.
(1) Write a couple of sentences describing what you find.
(2) Next, write a paragraph describing the activation energy and how it affects chemical reaction rates, and what its origins are.
Example 3-1
Calculate the activation energy for the decomposition of benzene diazonium chlo-ride to give chlorobenzene and nitrogen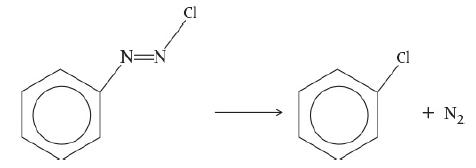
N=N Cl Cl + N
Step by Step Solution
3.40 Rating (150 Votes )
There are 3 Steps involved in it
a b Example 31 1 For lower activation energy we observe that the increase in ... View full answer

Get step-by-step solutions from verified subject matter experts


