Find the rate for the reaction of Problem 11. Data from Problem 11 Aqueous A at a
Question:
Find the rate for the reaction of Problem 11.
Data from Problem 11
Aqueous A at a concentration CA0 = 1 mol/liter is introduced into a batch reactor where it reacts away to form product R according to stoichiometry A → R. The concentration of A in the reactor is monitored at various times, as shown below:
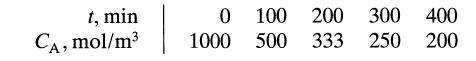 For CA0 = 500 mol/m3 find the conversion of reactant after 5 hours in the batch reactor.
For CA0 = 500 mol/m3 find the conversion of reactant after 5 hours in the batch reactor.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:





