The first-order reversible liquid reaction takes place in a batch reactor. After 8 minutes, conversion of A
Question:
The first-order reversible liquid reaction
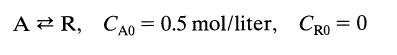
takes place in a batch reactor. After 8 minutes, conversion of A is 33.3% while equilibrium conversion is 66.7%. Find the rate equation for this reaction.
Transcribed Image Text:
AR, CAO = 0.5 mol/liter, CRO = 0
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (QA)
To find the rate equation for a firstorder reversible liquid reaction we can use the concept of reac...View the full answer

Answered By

Utsab mitra
I have the expertise to deliver these subjects to college and higher-level students. The services would involve only solving assignments, homework help, and others.
I have experience in delivering these subjects for the last 6 years on a freelancing basis in different companies around the globe. I am CMA certified and CGMA UK. I have professional experience of 18 years in the industry involved in the manufacturing company and IT implementation experience of over 12 years.
I have delivered this help to students effortlessly, which is essential to give the students a good grade in their studies.
3.50+
2+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Engineering questions
-
What is it like to be a minority within the majority culture? Here are some diversity-related scenarios that you, as a manager, might tackle. Diversity is a very touchy subject, so I hope our...
-
HealthySight is a manufacturer of high-quality lenses for sunglasses and ski goggles. HealthySight uses a standard process costing system and carries inventories at standard. In May 2001, the...
-
The second order liquid phase reaction C6H5COCH2Br+C6H5NC6H5COCH2NC5H5Br Is performed in a batch reactor at 35 oC. The specific reaction rate constant is 0.0445 dm3/mol/min. Reactor 1 is charged with...
-
Assume that the duration of human pregnancies can be described by a Normal model with mean 266 days and standard deviation 16 days. a) What percentage of pregnancies should last between 270 and 280...
-
Laura, a bond portfolio manager, administers a $10 million portfolio. The portfolio currently has a duration of 8.5 years. Laura wants to shorten the duration to 6 years using T-bill futures. T-bill...
-
Presented below is the pre-closing trial balance for the Scholarship Fund, a private-purpose trust fund of the Algonquin School District. Prepare the year-end closing entries and a Statement of...
-
What does a technician measure to set a proximity probe into a machine for the most useful range?
-
Open the Emoji Solution.sln file contained in the VB2017\Chap01\Emoji Solution folder. If necessary, open the designer window. The interface contains one label control and one button. You can use a...
-
17. The following data was obtained from orthogonal turning of PEEK and short- fiber-reinforced PEEK (C/PEEK, V = 0.3) [24]. Refer to Fig. 2.2 for definition of cutting variables. The cutting...
-
Aqueous A at a concentration C A0 = 1 mol/liter is introduced into a batch reactor where it reacts away to form product R according to stoichiometry A R. The concentration of A in the reactor is...
-
Aqueous A reacts to form R (A R) and in the first minute in a batch reactor its concentration drops from C A0 = 2.03 mol/liter to C Af = 1.97 mol/liter. Find the rate equation for the reaction if...
-
A regular polygon is an n-sided polygon in which all sides are of the same length and all angles have the same degree (i.e., the polygon is both equilateral and equiangular). The formula for...
-
You put $1,000 into a bank account today and then add $100 at the end of each of the next 5 years, with a 7% interest rate, how much will you have on the date of the last payment?
-
The fact that Apple has no manufacturing facilities of its own Question 21 options: has been problematic for Apple in terms of debt. has caused it to build up massive debt on its balance sheet. has...
-
More recently, studies have found evidence suggesting that many executives have engaged in a more direct form of manipulating their stock option compensation: backdating their option grants. What is...
-
Explain Statistical Process Control. What are the different types of variations?
-
Stocks are trading at 18.6X earnings. You expect earnings to grow 5.9% per year over the next five years. At the end of five years, you expect stocks to be trading at 20X earnings. The dividend yield...
-
Why are economies of scale central to the argument that monopolies may end up producing more and charging less than perfectly competitive firms?
-
A liquid flows upward through a valve situated in a vertical pipe. Calculate the differential pressure (kPa) between points A and B. The mean velocity of the flow is 4.1 m/s. The specific gravity of...
-
Go to the five LearnChemE screencasts link for Chapter 5 (http://www.umich.edu/~elements/6e/05chap/learn-cheme-videos.html). 1. In the screencast of the PBR with pressure drop, is there a problem...
-
How would you modify Table 6-2 for a. A constant-volume gas-phase reaction, and b. A variable-volume gas-phase reaction? Table 6-2 1. Mole balances: BR dNAdt=rAV dNBdt=rBV dNCdt=rCV dNDdt=rDV PFR PBR...
-
(a) Without referring back, make a list of the most important items you learned in this chapter. (b) Overall, what do you believe were the three major purposes of the chapter?
-
Amir has plans to gather several colleagues to establish a business institution. However, not all of his colleagues have capital in the form of money. Meanwhile, Anto is an activist in a village who...
-
respond to your classmates' posts on specialized trusts. Make sure you address a different type of trust then the one you discussed in your second post. If your second and third posts address the...
-
A preemptive right is the right of existing shareholders to maintain their proportion of ownership of a company . They do so by acquiring their proportional share of any additional stock issuances by...

Study smarter with the SolutionInn App


