Question: Repeat Example 24.6 with the following two changes Example 24.6 Figure E24.6 = We wish to lower the concentration of B in the liquid (V,
Repeat Example 24.6 with the following two changes
Example 24.6
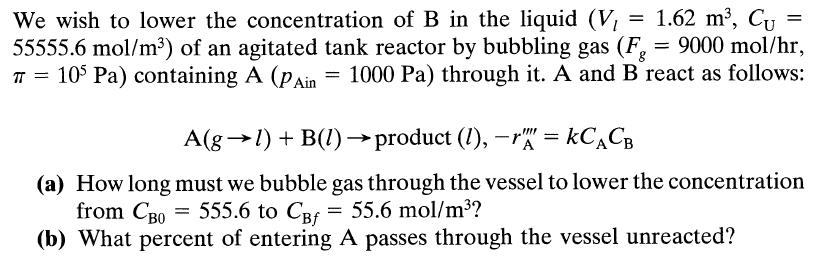
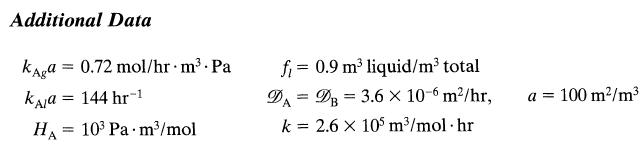
Figure E24.6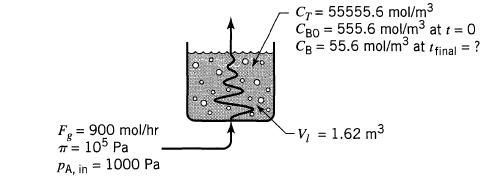

= We wish to lower the concentration of B in the liquid (V, 1.62 m, Cu = 55555.6 mol/m) of an agitated tank reactor by bubbling gas (Fg = 9000 mol/hr, T = 105 Pa) containing A (PAin = 1000 Pa) through it. A and B react as follows: A(gl) + B(1)product (1), -r = KCACB (a) How long must we bubble gas through the vessel to lower the concentration from CB0 = 555.6 to CBf = 55.6 mol/m? (b) What percent of entering A passes through the vessel unreacted?
Step by Step Solution
3.35 Rating (158 Votes )
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


