The catalytic reaction A B takes place within a fixed bed containing spherical porous catalyst X22.
Question:
The catalytic reaction A → B takes place within a fixed bed containing spherical porous catalyst X22. Figure P15-2B shows the overall rates of reaction at a point in the reactor as a function of temperature for various entering total molar flow rates, FT0.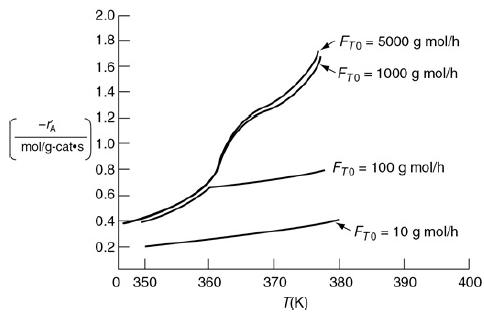
Reaction rates in a catalyst bed.
A graph plots negative r prime subscript A (mol per grams of catalyst times second) against Temperature T (in kelvin). The vertical axis represents the reaction rate ranging from 0 to 2 and the horizontal axis represents the temperature ranging from 0 to 400. The curves are drawn for the following flow rates (F subscript T0): 5000 gram mol per hour, 1000 gram mol per hour, 100 gram mol per hour, and 10 gram mol per hour. It is observed that for flow rate of 5000 and 1000 the curves show increasing trends with steeper slope and the curves for flow rate of 100 and 10 show constant or shallow increasing trends.
a. Is the reaction limited by external diffusion?
b. If your answer to part (a) was “yes,” under what conditions of those shown (i.e., T, FT0) is the reaction limited by external diffusion?
c. Is the reaction “reaction-rate-limited”?
d. If your answer to part (c) was “yes,” under what conditions of those shown (i.e., T, FT0) is the reaction limited by the rate of the surface reactions?
e. Is the reaction limited by internal diffusion?
f. If your answer to part (e) was “yes,” under what conditions of those shown (i.e., T, FT0) is the reaction limited by the rate of internal diffusion?
g. For a flow rate of 10 g mol/h, determine (if possible) the overall effectiveness factor, Ω = η, at 360 K.
h. Estimate (if possible) the internal effectiveness factor, η, at 367 K.
i. If the concentration at the external catalyst surface is 0.01 mol/dm3, calculate (if possible) the concentration at r = R/2 inside the porous catalyst at 367 K.
Additional information: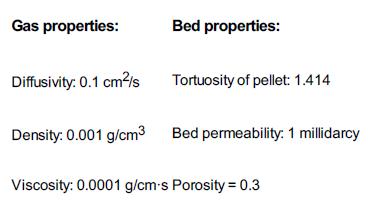
Step by Step Answer:






