The following reactions take place in a batch reactor. Additional information: a. Plot and analyze conversion and
Question:
The following reactions![]()
take place in a batch reactor.
Additional information: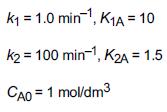
a. Plot and analyze conversion and the concentrations of A, D, and U as a function of time. When would you stop the reaction to maximize the concentration of D?
Describe what you find.
b. When does the maximum concentration of U occur?
c. What are the equilibrium concentrations of A, D, and U?
d. What would be the exit concentrations from a CSTR with a space time of 1.0 min? Of 10.0 min? Of 100 min?
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:





