The stoichiometry of a liquid-phase decomposition is known to be In a series of steady-state flow experiments
Question:
The stoichiometry of a liquid-phase decomposition is known to be
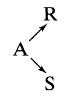
In a series of steady-state flow experiments (CA0 = 100, CRO = CSO = 0) in a laboratory mixed flow reactor the following results are obtained:

Further experiments indicate that the level of C, and Cs have no effect on the progress of the reaction.
With a feed CA0 = 100 and exit concentration CAf = 20, find CR at the exit from a plug flow reactor.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:





