We plan to remove about 90% of the A present in a gas stream by absorption in
Question:
We plan to remove about 90% of the A present in a gas stream by absorption in water which contains reactant B. Chemicals A and B react in the liquid as follows:![]()
B has a negligible vapor pressure, hence does not go into the gas phase. We plan to do this absorption in either a packed bed column, or an agitated tank contactor.
(a) What volume of contactor is needed?
(b) Where does the resistance of absorption reaction lie?
Data 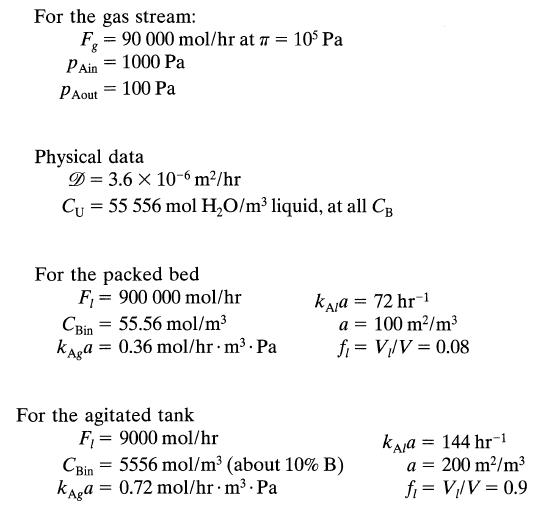
Note that Fl and CBin are very different in packed beds and tank contactors, and here is the reason why. Packed columns need Fl/Fg = 10 for satisfactory operations. This means large F,, and so as not to waste reactant B, it is introduced in low concentration. On the other hand, tank contactors do not have this flow restriction. Thus we can use low Fl and high CBin, as long as we introduce sufficient B to react with A.
Step by Step Answer:






