A 0.446-g sample of an unknown monoprotic acid is titrated with 0.105 M KOH. The resulting titration
Question:
A 0.446-g sample of an unknown monoprotic acid is titrated with 0.105 M KOH. The resulting titration curve is shown here.
Determine the molar mass and pKa of the acid.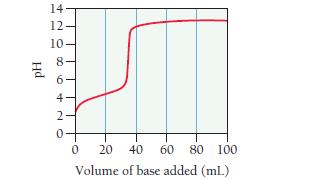
Transcribed Image Text:
Hd 14 12- 10- ∞06+NO 8- 4- 2 20 40 60 80 100 Volume of base added (mL) 0
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (2 reviews)
To determine the molar mass of the acid 1 Calculate the moles of KOH used in the titration 2 Since t...View the full answer

Answered By

Shaira grace
I have experience of more than ten years in handing academic tasks and assisting students to handle academic challenges. My level of education and expertise allows me communicate eloquently with clients and therefore understanding their nature and solving it successfully.
5.00+
4+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
A 0.229-g sample of an unknown monoprotic acid is titrated with 0.112 M NaOH. The resulting titration curve is shown here. Determine the molar mass and pK a of the acid. Hd 14 12- 10 8 6- Na 00 4- 0...
-
A 0.5224-g sample of an unknown monoprotic acid was titrated with 0.0998 M NaOH. The equivalence point of the titration occurred at 23.82 mL. Determine the molar mass of the unknown acid.
-
Why " Kodak " is unsuccessful in implementing a strategy. Can you prepare a critical examination of the strategy to address the following questions about Kodak. What was the strategy and why do you...
-
Winters Inc. has been manufacturing its own shades for its table lamps. The company is currently operating at 100% of capacity. Variable manufacturing overhead is charged to production at the rate of...
-
The following is a list of well-known companies. 1. H&R Block 2. eBay Inc. 3. Wal-Mart Stores, Inc. 4. Ford Motor Company 5. Citigroup 6. Boeing 7. SunTrust 8. Alcoa Inc. 9. Procter & Gamble 10....
-
How many forms of main() are there?
-
Pantanal, Inc., manufactures car seats in a local factory. For costing purposes, it uses a first-in, first-out (FIFO) process costing system. The factory has three departments: Molding, Assembling,...
-
3. If the policy proposed in question 2 is adopted, analyze the potential effects of this policy on the demand for workers under the age of 65. That is, if employers who want their employees to be...
-
A 20.0-mL sample of 0.115 M sulfurous acid (H 2 SO 3 ) solution is titrated with 0.1014 M KOH. At what added volume of base solution does each equivalence point occur?
-
A 25.0-mL sample of 0.125 M pyridine is titrated with 0.100 M HCl. Calculate the pH at each volume of added acid: 0 mL, 10 mL, 20 mL, equivalence point, one-half equivalence point, 40 mL, 50 mL....
-
Name three alternative types of email marketing that can be used for customer acquisition.
-
Below is an alphabetical list of Terrier Company's December 31, 2022 balance sheet accounts and amounts. Prepare a properly formatted, classified balance sheet for Terrier Company. You may prepare...
-
In what ways is social stratification both constructed and perpetuated, while concurrently manifesting through mechanisms of privilege and/or oppression in the lived experiences of individuals within...
-
Describe how quantitative and qualitative data are treated differently in analysis. What kind of questions are qualitative and quantitative data well suited to address? Explain, with examples.
-
Consider the following circuit CLK QA CLK O 1 K J 9 Q 16 K 15 QB 30 J Q CLK K k " o QD 1. Give the equations for the inputs J, K of the flip flops 2. We suppose that the initial value of the...
-
What is an economic argument in favor of more humane border policies regarding migrants and refugees?
-
Find the volume of the solid bounded by the cylinder y^2 + z^2 = 4 and the planes x = 2y, x = 0, z = 0 in the first octant. Calculate the double integral by using two iterated integrals with...
-
Show that, given a maximum flow in a network with m edges, a minimum cut of N can be computed in O(m) time.
-
A glass camera lens with an index of 1.55 is to be coated with a cryolite film (n 1.30) to decrease the reflection of normally incident green light ( 0 = 500 nm). What thickness should be deposited...
-
Using Fig. 9.73, which depicts the geometry of the Shuttle radar interferometer, show that z(x) = h - r 1 cos θ Then use the Law of Cosines to establish that Eq. (9.108) is correct. Fig....
-
Given that the mirrors of a FabryPerot Interferometer have an amplitude reflection coefficient of r = 0.894 4, find (a) The coefficient of finesse, (b) The half-width, (c) The finesse, and, (d) The...
-
How did Hauxi village manage to become so successful and when comparing it to major cities in China and other countries like north Korea and U.S.A w that would the differences be?
-
Suppose the original price for the tree products are $1k Model S: P=100 Model 3 P=45 Model Y P=55 1. Based on your estimated demand functions, compute the own price elastic of demand for each car at...
-
What are the implications for Dow from the following provisions in the merger agreement a) closing conditions? Why do you think Dow agreed to these provisions? Closing Conditions 6.1, 6.2: Conditions...

Study smarter with the SolutionInn App


