A base is known to be one of the three listed in the table. You are given
Question:
A base is known to be one of the three listed in the table. You are given a sample of the base and asked to identify it. To do so, you dissolve 0.30 g of the base in enough water to make 25.0 mL of the basic solution. You then titrate the solution with 0.100 M HCl and record the pH as a function of the added acid resulting in the titration curve that follows. Examine the table and the titration curve and answer the questions.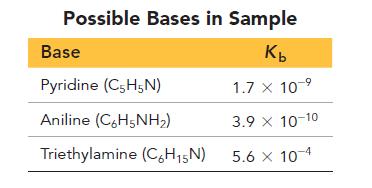
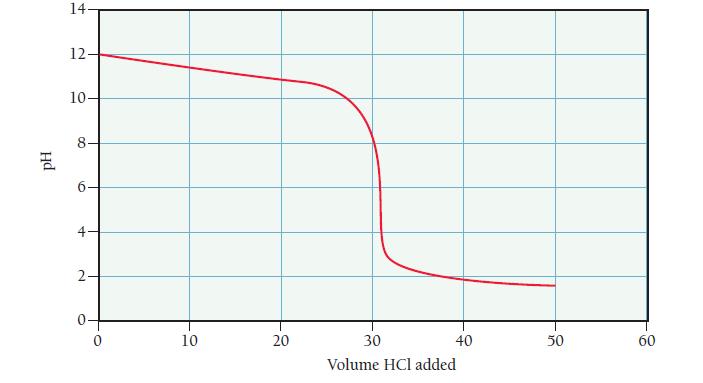
Titration Curve for 0.30 g of Unknown Base Dissolved in 25.0 mL of Solution
a. What is the volume of added HCl at the equivalence point?
b. What is the pH at the half-equivalence point?
c. What is the molar mass of the unknown base?
d. What are the pKb and Kb of the unknown base?
e. What is the most likely identity of the unknown base?
Step by Step Answer:






