A chemist decomposes several samples of carbon monoxide into carbon and oxygen and weighs the resultant elements.
Question:
A chemist decomposes several samples of carbon monoxide into carbon and oxygen and weighs the resultant elements.
The results are shown in the table.
a. Do you notice a pattern in these results?
Next, the chemist decomposes several samples of hydrogen peroxide into hydrogen and oxygen. The results are shown in the table.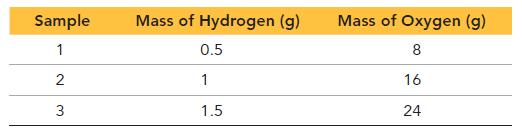
b. Do you notice a similarity between these results and those for carbon monoxide in part a?
c. Can you formulate a law from your observations in a and b?
d. Can you formulate a hypothesis that might explain your law in c?
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:





