Balance each redox reaction occurring in acidic aqueous solution. a. K(s) + Cr+ (aq). Cr(s) + K+
Question:
Balance each redox reaction occurring in acidic aqueous solution.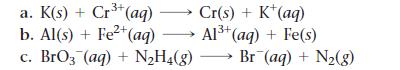
Transcribed Image Text:
a. K(s) + Cr³+ (aq). Cr(s) + K+ (aq) b. Al(s) + Fe²+ (aq) Al³+ (aq) + Fe(s) c. BrO3(aq) + N₂H₂(g) →→→ Br¯(aq) + N₂(g)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 50% (2 reviews)
a 3 Ks Cr aq Crs 3 ...View the full answer

Answered By

Charles mwangi
I am a postgraduate in chemistry (Industrial chemistry with management),with writing experience for more than 3 years.I have specialized in content development,questions,term papers and assignments.Majoring in chemistry,information science,management,human resource management,accounting,business law,marketing,psychology,excl expert ,education and engineering.I have tutored in other different platforms where my DNA includes three key aspects i.e,quality papers,timely and free from any academic malpractices.I frequently engage clients in each and every step to ensure quality service delivery.This is to ensure sustainability of the tutoring aspects as well as the credibility of the platform.
4.30+
2+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
PART A: Balance each redox reaction occurring in acidic aqueous solution. MnO4( a q )+Al( s )Mn2+( a q )+Al3+( a q ). Express your answer as a chemical equation. Identify all of the phases in your...
-
Balance each redox reaction occurring in acidic aqueous solution. a. I (aq) + NO (aq) b. CIO4 (aq) + Cl(aq) c. NO3(aq) + Sn+ (aq) 1(s) + NO(g) CIO3(aq) + Cl(g) Sn+ (aq) + NO(g)
-
Balance each redox reaction occurring in acidic aqueous solution. a. PbO(s) + I (aq) Pb+ (aq) + 1(s) 2+ b. SO32 (aq) + MnO4 (aq) SO42 (aq) + Mn+ (aq) 2- c. S03 (aq) + Cl(g) SO4 (aq) + Cl (aq)
-
4) Solve the following problems: 9 (b) 1. Apply the DFS based topological sort algorithm to the graph on the left; Your DFS traversal will start with vertext a. Write down the nodes visited during a...
-
You are the production manager for Great Grain Corporation, a manufacturer of four cereal products. The companys best-selling product is Smackaroos, a sugar-coated puffed rice cereal. Yesterday,...
-
The year-end balance sheet of Helsinki Corporation, a wholly owned British affiliate in Finland, is reproduced here. Relevant exchange rate and inflation information is also provided. Required: Using...
-
Do the following activities to complete your marketing plan: 1. Draw a simple organizational chart for your organization. 2. Develop a Gantt chart (see Chapter 2) to schedule the key activities...
-
Renner Hardware Store completed the following merchandising transactions in the month of May. At the beginning of May, the ledger of Renner showed Cash of $5,000 and Owners Capital of $5,000. May 1...
-
what is meant by the term functionality of a database management system? Explain the responsibility of any two functionalities of a database management system. Discuss how a database transaction is...
-
How can the corrosion of iron be prevented?
-
Explain the role of each of the following in promoting corrosion: moisture, electrolytes, and acids.
-
Two commercial airplanes are flying at an altitude of 40,000 ft along straight-line courses that intersect at right angles. Plane A is approaching the intersection point at a speed of 442 knots...
-
Guitano, age 67, is concerned about retirement income for his spouse, Marianne, age 66, should he predecease her. He currently receives 100% of the maximum CPP retirement pension. Marianne has a CPP...
-
Two tempered-steel bars, each 3/16 in. thick, are bonded to a 1/2-in. mild-steel bar. This composite bar is subjected as shown to a centric axial load of magnitude P. Both steels are elastoplastic...
-
Dave and Kathy, both age 58, own and operate an antique shop that they plan to run it for as long as possible and then pass along to the family. The business is worth $500,000 and the couple's other...
-
You make a one-time investment of pre-income-tax funds of $5,000 in each of the following accounts for 35 years, earning 10% per year. Assume that all your tax rates stay at 20% over time. How much...
-
58. Allyza is employed in JBC Corporation. She has the following for the current year: Statutory minimum wage Overtime pay Night-shift differential Commission from the same employer Total How much is...
-
Suppose that the current price of oil is $60 per barrel and the quantity sold is 90 million barrels per day. Assume that the supply and demand curves for oil are linear. The current estimates of the...
-
Danielle has an insurance policy with a premium of $75 per month. In September she is in an accident and receives a bill worth $2990 for the repair of her own property. Her deductible is $250 and her...
-
Add a new column to Table 10.1, listing the specific gravity of each substance. TABLE 10.1 Densities of Some Common Solids, Liquids, and Gases Density (kg/m) Substance SOLIDS Ice (at 0C) 917 Aluminum...
-
The region near the South Pole in Antarctica has an altitude of about 3000 m, making the air pressure lower than at sea level. Explain why it is more difficult for an airplane to take off from the...
-
Find the pressure at the bottom of a swimming pool that is 2.5 m deep.
-
Direct Labor Budget Duran Company produces asphalt roofing materials. The production budget in bundles for Duran's most popular weight of asphalt shingle is shown for the following months: Units...
-
Novelli's Nursery has developed the following data in order to calculate the lower of cost or net realizable value for its products. The individual products are listed within the categories of trees....
-
Flounder Company makes swimsuits and sells these suits directly to retailers. Although Flounder has a variety of sults, it does not make the Performance suit used by highly skilled swimmers. The...

Study smarter with the SolutionInn App


