Balance each redox reaction occurring in basic aqueous solution. a. HO(aq) + ClO(aq) CIO (aq) + O(g)
Question:
Balance each redox reaction occurring in basic aqueous solution.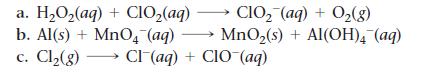
Transcribed Image Text:
a. H₂O₂(aq) + ClO₂(aq) CIO₂ (aq) + O₂(g) b. Al(s) + MnO4 (aq) → MnO₂(s) + Al(OH)4 (aq) c. Cl₂(g) →→→ Cl(aq) + CIO (aq)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 50% (2 reviews)
a HOaq 2 C1Oaq 2 OHaq ...View the full answer

Answered By

Sourindra Samanta
I'm teaching students as private tutor from last 2 years
Teaching math & science & advance biology in higher level
0.00
0 Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
PART A: Balance each redox reaction occurring in acidic aqueous solution. MnO4( a q )+Al( s )Mn2+( a q )+Al3+( a q ). Express your answer as a chemical equation. Identify all of the phases in your...
-
Balance each redox reaction occurring in basic aqueous solution. a. MnO4 (aq) + Br (aq) MnO(s) + BrO3(aq) b. Ag(s) + CN- (aq) + O(g) Ag(CN) (aq) c. NO (aq) + Al(s) - NH3(g) + AlO (aq)
-
Balance each redox reaction occurring in acidic aqueous solution. a. I (aq) + NO (aq) b. CIO4 (aq) + Cl(aq) c. NO3(aq) + Sn+ (aq) 1(s) + NO(g) CIO3(aq) + Cl(g) Sn+ (aq) + NO(g)
-
Gabriele Enterprises has bonds on the market making annual payments, with seven years to maturity, a par value of $1,000, and selling for $974. At this price, the bonds yield 7.2 percent. What must...
-
Suppose you are a management consultant and a client asks you why companies include standard costs in their cost accounting systems. Prepare your response, listing several purposes for using standard...
-
Gray proposed a framework linking culture and accounting. He predicts four accounting values (professionalism, uniformity, conservatism, and secrecy) based on Hofstedes four cultural dimensions...
-
1. To develop an understanding of your ethical leadership style 2. To understand how your preferred ethical leadership style relates to other ethical leadership styles Directions 1. Please read the...
-
Blueline Tours, Inc., operates tours throughout the United States. A study has indicated that some of the tours are not profitable, and consideration is being given to dropping these tours to improve...
-
Compare and contrast benefits and challenges that exist between centralized database management systems and distributed database management systems. Identify potential business environments where...
-
Sketch a voltaic cell for each redox reaction. Label the anode and cathode and indicate the half-reaction that occurs at each electrode and the species present in each solution. Also indicate the...
-
Balance each redox reaction occurring in acidic aqueous solution. a. PbO(s) + I (aq) Pb+ (aq) + 1(s) 2+ b. SO32 (aq) + MnO4 (aq) SO42 (aq) + Mn+ (aq) 2- c. S03 (aq) + Cl(g) SO4 (aq) + Cl (aq)
-
In Chapter 34, we will model particle diffusion as a random walk in one dimension. In such processes, the probability of moving an individual step in the +x or x direction is equal to one half....
-
Crystal invested $245,000 to purchase a home. After 11 years, he sold the home for $335,000. Calculate the effective interest rate earned on this investment. % Round to two decimal places
-
Rosie's cupcakes has sales of $256,000, costs of goods sold of $189,000, average accounts receivable of $28,800, and average accounts payable of $89,600. How long does it take for the firm's credit...
-
Write an update statement to remove the term field from documents that the value of the term filed is 3.
-
If log x-y - 10 (logx+logy), show that x + y = 11xy. 3
-
Yolanda bought a vacation home for $255,000, plus $500 in legal fees. She also paid an additional closing cost of $4,500 to cover the cost of a survey, title search, and recording fees. She also gave...
-
Corporation XYZ has the following data on labor input and production of commodity Z for each of the eight production periods. 1. Use the data on labor input and total output to calculate the marginal...
-
A summary of changes in Pen Corporation's Investment in Sam account from January 1, 2011, to December 31, 2013, follows (in thousands): ADDITIONAL INFORMATION 1. Pen acquired its 80 percent interest...
-
A suction cup works by virtue of a vacuum that is created within the cup. When the cup is pressed against a flat surface, most of the air is forced out, leaving a region of very low pressure. If a...
-
Two blocks with the same volume but different densities are in a lake as shown in Figure Q10.14. Block m 1 floats at the surface with a portion above the water level, and block m 2 floats underwater....
-
The four tires on the authors car are inflated to an absolute pressure of 2.5 10 5 Pa. If the car has a mass of 1800 kg, what is the contact area between each tire and the ground?
-
The risk-free rate is 2 percent, the expected return on the PSEi is 14 percent, and its standard deviation is 23 percent. A local equity ABC stock has a standard deviation of 51 percent but is...
-
An insurance company is trying to sell you an investment product that will generate $5,000 per year forever from the next year (t = 1). If the discount rate for this investment is 14%, how much would...
-
Grackle Corporation, not a personal service corporation, had $240,000 ofnet active income, $30,000 of portfolio income, and a $250,000 passive activity loss during the year. How much is Grackle's...

Study smarter with the SolutionInn App


