Calculate H rxn for the reaction: Use the following reactions and given Hs: CaO(s) + CO2(g) CaCO3(s)
Question:
Calculate ΔHrxn for the reaction:![]()
Use the following reactions and given ΔH’s: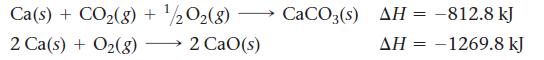
Transcribed Image Text:
CaO(s) + CO2(g) CaCO3(s)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (2 reviews)
To calculate Hrxn for the reaction shown in the image we can use the followi...View the full answer

Answered By

Bhartendu Goyal
Professional, Experienced, and Expert tutor who will provide speedy and to-the-point solutions. I have been teaching students for 5 years now in different subjects and it's truly been one of the most rewarding experiences of my life. I have also done one-to-one tutoring with 100+ students and help them achieve great subject knowledge. I have expertise in computer subjects like C++, C, Java, and Python programming and other computer Science related fields. Many of my student's parents message me that your lessons improved their children's grades and this is the best only thing you want as a tea...
3.00+
2+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
A builder gets a construction loan of 10 million. For simplicity, assume the loan has an interest rate at j1=6%. The loan does not require payment during the construction process. Construction will...
-
What are the advantages and disadvantages of organizing a business as a corporation? 2. How does a partnership differ from a limited liability company? 3. Why do corporates file for bankruptcy? 4....
-
Calculate S values for the following reactions by using tabulated values from Appendix C. In each case explain the sign of S. (c) HNO3(g) NH3 (g)- NH4NO3(s) 2 Fe203(s)4 Fe(s) 302(g) CaCO3(s,calcite)...
-
Stenback Exercise Equipment, Inc. reported the following financial statements for 2016: Requirements 1. Compute the amount of Stenback Exercises acquisition of plant assets. Assume the acquisition...
-
At April 30 the bank reconciliation of Guardado Company shows three outstanding checks: No. 254 $650, No. 255 $700 and No. 257 $410. The May bank statement and the May cash payments journal are given...
-
Distinguish between P-time and M-time.
-
What sources does an auditor use to test for overstated accounts payable, and how are those sources used?
-
Presented below are selected transactions of Molina Company. Molina sells in large quantities to other companies and also sells its product in a small retail outlet. March 1 Sold merchandise on...
-
Required Compute variances for the following items and indicate whether each variance is favorable (F) or unfavorable (U): Note: Select "None" if there is no effect (i.e., zero variance). Item Sales...
-
In Figure 12.1, let region 2 be free space, while μ r1 = 1, " r1 = 0, and ' r1 is unknown. Find ' r1 if (a) The amplitude of E 1 is one-half that of E + 1 ; (b) (S 1 ) is one-half of (S...
-
Calculate H rxn for the reaction: Use the following reactions and given Hs: CH4(g) + 4 Cl2(g) CC14(g) + 4 HCl(g)
-
Calculate H rxn for the reaction: Use the following reactions and given Hs: FeO3(s) + 3 CO(g) 2 Fe(s) + 3 CO2(8)
-
Describe the basic factors that influence the selection of a project organizational form, and their influences. Compare and contrast the role of the functional manager and the project manager in...
-
Can you elucidate the symbiotic relationship between autonomy and collaboration in innovative projects?
-
Why does it later rise? How would you describe that in economic terms again?
-
Paul corporation of profit, maximizing monopoly. It sells a patented rabies vaccine for pets and earns economic profits.
-
here are 4 common categories of customers: new, existing, exiting, and exited. Suppose you work for a large financial institution that is looking to improve its savings deposits and retirement...
-
Q. Today, social, mobile, and local marketing are the fastest-growing forms of online marketing. This assignment is to plan your own business. A. Describe its business model mentioning the comparison...
-
Look up the table of contents for the responsible business reports of a company of your choice and rewrite it by integrating new social, environmental, and ethics considerations. Then describe the...
-
Write the binomial probability in words. Then, use a continuity correction to convert the binomial probability to a normal distribution probability. P(x 110)
-
Singlet carbenes add to alkenes to yield cyclopropanes. Stereochemistry is maintained, meaning that cis- and trans-substituted alkenes give cis- and trans-substituted cyclopropanes, respectively; for...
-
Some symmetry operations can be carried out physically using a ball-and-stick model of a molecule without disassembly and reassembly and others can only be imagined. Give two examples of each...
-
Predict the number of chemically shifted 1 H peaks and the multiplet splitting of each peak that you would observe for 1,1,1,2-tetrachloroethane. Justify your answer. I H1 --c-CI CI | Cl
-
At one instant, a 2.0 kg particle has position vector =(4.0+6.0) m and acceleration vector =(5.02.0) m/s2 . Just then, what is the torque (Nm) on the particle?
-
Lessee Company enters into a 6 - year finance lease of non - specialized equipment with Lessor Company on January 1 . Lessee has agreed to pay $ 2 8 , 0 0 0 annually beginning immediately on January...
-
Two particles move along an x axis. The position of particle 1 is given by x = 8.00 t 2 + 7.00 t + 6.00 (in meters and seconds); the acceleration of particle 2 is given by a = -10.0 t (in meters per...

Study smarter with the SolutionInn App


