Calculate how many moles of NH 3 form when each quantity of reactant completely reacts. 3 NH4(1)
Question:
Calculate how many moles of NH3 form when each quantity of reactant completely reacts.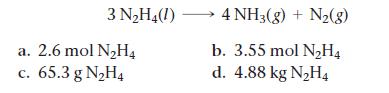
Transcribed Image Text:
3 N₂H4(1) a. 2.6 mol N₂H4 c. 65.3 g N₂H4 4 NH3(g) + N₂(8) b. 3.55 mol N₂H4 d. 4.88 kg N₂H4
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 75% (4 reviews)
To calculate the moles of NH3 formed when each quantity of NH completely reacts we can use the balan...View the full answer

Answered By

Stephen ouma
I have worked with different academic writing companies such as wriredom, writerbay, and Upwork. While working with these companies, I have helped thousands of students achieve their academic dreams. This is what I also intend to do here in SolutionInn
4.90+
19+ Reviews
63+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Calculate how many moles of NO 2 form when each quantity of reactant completely reacts. 2 NO5(g) a. 2.5 mol NO5 b. 6.8 mol NO5 c. 15.2 g NO5 d. 2.87 kg NO5 4 NO(g) + O(g)
-
Aluminum hydroxide reacts with sulfuric acid as follows: Which is the limiting reactant when 0.500 mol Al(OH)3 and 0.500 mol H2SO4 are allowed to react? How many moles of Al2(SO4)3 can form under...
-
A mixture of N2(g) and H2(g) reacts in a closed container to form ammonia, NH3(g). The reaction ceases before either reactant has been totally consumed. At this stage 3.0 mol N2, 3.0 mol H2, and 3.0...
-
Corning-Howell reported taxable income in 2013 of $120 million. At December 31, 2013, the reported amount of some assets and liabilities in the financial statements differed from their tax bases as...
-
In comparing accounting net income and operating cash flow, what two items do you find in net income that are not in operating cash flow? Explain what each is and why it is excluded in operating cash...
-
Discuss the risks auditors face by not following professional auditing standards and how those risks are expected to change in the near future as a result of changes in business conditions,...
-
All the spontaneous processes are (a) Reversible (b) Irreversible (c) Quasi-static (d) None of these.
-
How could this fraud have been prevented? Why is this a difficult fraud to prevent? May 13, 1988, a Friday incidentally, will be remembered by a major Chicago bank. Embezzlers nearly escaped with $69...
-
ances Rundle Manufacturing Company was started on January 1, 2018, when it acquired $76,000 cash by Issuing common stock. Rundle Immediately purchased office furniture and manufacturing equipment...
-
Summarize sales by month and sales division using a pivot table in Excel (hint: put divisions in columns, months in rows) or using a graph or chart in Tableau. If you are using Excel to summarize,...
-
Consider the balanced equation: Complete the table showing the appropriate number of moles of reactants and products. If the number of moles of a reactant is provided, fill in the required amount of...
-
Consider the unbalanced equation for the neutralization of acetic acid: Balance the equation and determine how many moles of Ba(OH) 2 are required to completely neutralize 0.461 mole of HC 2 H 3 O 2...
-
Determine whether the statement is true or false. If it is true, explain why. If it is false, explain why or give an example that disproves the statement. '4 dx = In 15 x? 1
-
As more developing countries are moving into the middle-income group, few graduates to develop country status (high-income group). The developing countries can get 'stuck in the middle' or they are...
-
What was Blossom earning per share? Blossom Corporation reports the following information: Net income $745000 Dividends on common stock $205000 Dividends on preferred stock $85000 Weighted-average...
-
Suppose demand and supply are given by Qx=14 (1/2)P and Q = (1/4)Px 1 Instructions: Enter your responses rounded to the nearest whole number. a. Determine the equilibrium price and quantity. Show the...
-
Summarize in different words the the following Over one billion people around the world live on less than $1 a day. In 2006, the median income for an individual in the United States was over $75 per...
-
Explain tonicity and how plant and animal cells deal with varying tonicity in their environments .
-
Name and briefly describe the five major historical themes of public relations through the centuries.
-
The overall reaction and equilibrium constant value for a hydrogenoxygen fuel cell at 298 K is 2H 2 (g) + O 2 (g) 2H 2 O(l) K = 1.28 10 83 a. Calculate E cell and G 8 at 298 K for the fuel cell...
-
Is the equation A = U TS applicable to all processes?
-
Under what conditions is K x > K P ?
-
Rank the following compounds in order of increasing reactivity toward electrophilic aromatic substitution: Br Br- Br
-
For the elements and summary of the law for common law marriages, apply it to the facts below. Facts: Mandy Johnson and Carl Lawrence met in middle school and fell in love. Mandy was two years older...
-
Each student will be assigned to a group to complete the crime prevention presentation.The presentation will explain the theory of crime prevention and discuss the three approaches to crime...
-
A uniformly charged spherical shell has inner and outer radii of 29.0 cm and 32.0 cm, respectively. A point charge of -45.0 nC is located at the center of the spherical shell. A proton orbits around...

Study smarter with the SolutionInn App


