Calculate K c for each reaction. a. [(g) = 21(g) Kp 6.26 x 10-22 (at 298 K)
Question:
Calculate Kc for each reaction.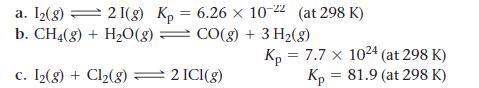
Transcribed Image Text:
a. [₂(g) = 21(g) Kp 6.26 x 10-22 (at 298 K) b. CH4(g) + H₂O(g) = CO(g) + 3 H₂(g) c. I₂(g) + Cl₂(g) = 2 ICI(g) = Kp = 7.7 x 1024 (at 298 K) Kp = 81.9 (at 298 K) P
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 40% (5 reviews)
a 256 ...View the full answer

Answered By

JAPHETH KOGEI
Hi there. I'm here to assist you to score the highest marks on your assignments and homework. My areas of specialisation are:
Auditing, Financial Accounting, Macroeconomics, Monetary-economics, Business-administration, Advanced-accounting, Corporate Finance, Professional-accounting-ethics, Corporate governance, Financial-risk-analysis, Financial-budgeting, Corporate-social-responsibility, Statistics, Business management, logic, Critical thinking,
So, I look forward to helping you solve your academic problem.
I enjoy teaching and tutoring university and high school students. During my free time, I also read books on motivation, leadership, comedy, emotional intelligence, critical thinking, nature, human nature, innovation, persuasion, performance, negotiations, goals, power, time management, wealth, debates, sales, and finance. Additionally, I am a panellist on an FM radio program on Sunday mornings where we discuss current affairs.
I travel three times a year either to the USA, Europe and around Africa.
As a university student in the USA, I enjoyed interacting with people from different cultures and ethnic groups. Together with friends, we travelled widely in the USA and in Europe (UK, France, Denmark, Germany, Turkey, etc).
So, I look forward to tutoring you. I believe that it will be exciting to meet them.
3.00+
2+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
For the reaction at 700°C, Kc = 0.534. Calculate the number of moles of H2 that are present at equilibrium if a mixture of 0.300 mole of CO and 0.300 mole of H2O is heated to 700°C in a...
-
How is HRM technology being used to "strategically" recruit and staff? Share an example. How does screening software present challenges for recruitment and staffing? How does staffing HRM technology...
-
The equilibrium constant for the reaction Is Kc = 1.3 Ã 10-2 at 1000 K. (c) Calculate Kc for NOBr(g) NO(g) + ½ Br2(g)?
-
Halemav paid $2 in dividends for each share over the past year and the company is not expected to increase its dividend distribution in the future. The risk free rate is 2% and the risk premium on...
-
Hampton Company is considering an investment in equipment that is capable of producing electronic parts twice as fast as existing technology. The outlay required is $2,340,000. The equipment is...
-
Two twins travel away from each other at relativistic speed. The time-dilation result from relativity says that each twin sees the others clock running slow, so each says the other has aged less. How...
-
Consider the following cash flow profile and assume MARR is 10 percent/year and the finance rate is 4 percent/year. a. Determine the MIRR for this project. b. Is this project economically attractive?...
-
Selected information from the payroll register of Anderson's Dairy for the week ended July 7, 20--, is shown below. The SUTA tax rate is 5.4%, and the FUTA tax rate is 0.8%, both on the first $7,000...
-
2. (7pts) Give an example of a 44 matrix A which have the following properties: (a) A is lower triangular. All its entries are integers. (b) A has two distinct eigenvalues. Each eigenvalue is a digit...
-
Calculate K p for each reaction. a. NO4(8) = 2 NO(g) b. N(g) + 3 H(g) = 2 NH3(g) c. N(g) + O(g) 2 NO(g) K = 5.9 x 10- (at 298 K) K = 3.7 x 108 (at 298 K) K = 4.10 x 10-1 (at 298 K)
-
Use the reactions and their equilibrium constants to predict the equilibrium constant for the reaction 2 A(s) 3 D(g). A(s) = 3 D(g) B(g) + C(g) B(g) + 2 C(g) K = 0.0334 K = 2.35
-
Gives a formula for a function y = (x) and shows the graphs of and -1 . Find a formula for -1 in each case. - 0 y f(x) = x 2x + 1, x 1 y = f-(x) 1 y = f(x) X
-
How is quality determined in healthcare? How is clinical quality different from service quality?
-
Why is alignment of employees perceptions critical for proper strategic action to take place? Why do you think executives perceptions might differ from those of other staff?
-
What is the value of gathering data over time and trending them?
-
What factors affect the magnitude of the time value of money?
-
How are products and services in healthcare different from one another?
-
Tercer reports the following on one of its products. Compute the direct materials price and quantity variances. Direct materials standard (4 lbs. @ $ 2/ lb.) . . . . . . . . $ 8 per finished unit...
-
Write the given system without the use of matrices. D) - ()- d (x sin t + 8 (2+ 1)
-
An ammeter model consists of an ideal ammeter in series with a 20-Ω resistor. It is connected with a current source and an unknown resistor R x as shown in Fig. 2.133 . The ammeter...
-
Design a circuit that uses a dArsonval meter (with an internal resistance of 2 k that requires a current of 5 mA to cause the meter to deflect full scale) to build a voltmeter to read values of...
-
The potentiometer (adjustable resistor) R x in Fig. 2.126 is to be designed to adjust current ix from 10 mA to 1 A. Calculate the values of R and R x to achieve this. Rx 110 V
-
How you book air travel and other travel products will depend on: your travel specialty and the products you sell, whether or not you have chosen to sell air travel (independent of tours and/or...
-
Only need help and answers for PART 3 Only. THE CLIENT: Background information! The Charlotte Hornets continue to have a tremendous following since rebranding from the Charlotte Bobcats back to the...
-
Identify FOUR examples of possible barriers to communication in the workplace, and explain how you would overcome these barriers.

Study smarter with the SolutionInn App


