Use the reactions and their equilibrium constants to predict the equilibrium constant for the reaction 2 A(s)
Question:
Use the reactions and their equilibrium constants to predict the equilibrium constant for the reaction 2 A(s) ⇌3 D(g).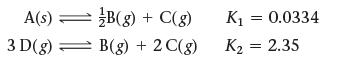
Transcribed Image Text:
A(s) = 3 D(g) B(g) + C(g) B(g) + 2 C(g) K₁ = 0.0334 K₂ = 2.35
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (2 reviews)
To predict the equilibrium constant for the reaction 2As 3 Dg we can use ...View the full answer

Answered By

Muhammad Umair
I have done job as Embedded System Engineer for just four months but after it i have decided to open my own lab and to work on projects that i can launch my own product in market. I work on different softwares like Proteus, Mikroc to program Embedded Systems. My basic work is on Embedded Systems. I have skills in Autocad, Proteus, C++, C programming and i love to share these skills to other to enhance my knowledge too.
3.50+
1+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Consider the reactions and their respective equilibrium constants: Use these reactions and their equilibrium constants to predict the equilibrium constant for the following reaction: NO(g) + Br(g) ...
-
If CIF value is RMB 10000, calculate how much is tariff 1.Including tariff, C.T and V.A.T (1) tariff 6%, C.T 30% = ( ) (2) tariff 14%, C.T 25% = ( ) (3) tariff 70%, C.T 15% = ( ) (4) tariff 120%, C.T...
-
Carbon monoxide replaces oxygen in oxygenated hemoglobin according to the reaction: a. Use the reactions and associated equilibrium constants at body temperature given here to find the equilibrium...
-
A person who is 6 feet and 3 inches tall and weighs 185 pound force (lbf) is driving a car at a speed of 65 miles per hour over a distance of 25 miles between two cities. The outside air temperature...
-
Each of the following scenarios is independent. All cash flows are after-tax cash flows. Required: 1. Tada Corporation is considering the purchase of a computer-aided manufacturing system. The cash...
-
The reliability of a hard-disk drive is typically described in terms of a quantity called mean time between failures (MTBF). Although this quantity is called a time, the MTBF actually is measured in...
-
What is Lean Thinking?
-
The Glory Mountain State Ski Are expects to attract 292,500 skier days during the coming ski season. A skier day represents one skier at the mountain for one day. In addition to a $ 2,000,000 per...
-
-S Data concerning a recent period's activity in the Prep Department, the first processing department in a company that uses process costing, appear below: Equivalent units in ending work in process...
-
Calculate K c for each reaction. a. [(g) = 21(g) Kp 6.26 x 10-22 (at 298 K) b. CH4(g) + HO(g) = CO(g) + 3 H(g) c. I(g) + Cl(g) = 2 ICI(g) = Kp = 7.7 x 1024 (at 298 K) Kp = 81.9 (at 298 K) P
-
This reaction has an equilibrium constant of Kp = 2.2 * 10 6 at 298 K. Calculate Kp for each reaction and predict whether reactants or products will be favored at equilibrium. 2 COF2(g) = CO(g) +...
-
What are the types of active air solar systems and how do they function?
-
Retained Earnings Balance (before Net Income) Paid-in Capital in Excess of Par Totals 80,690 82,690 $12,352,970 $12,352,970 -/50 !!! Prepare a balance sheet at December 31, 2025, for Novak...
-
You and a friend are walking towards your bus stop when her boy friend stops her to ask about the weekend. You keep walking at a constant velocity of 0 . 9 4 m / s and reach the bus 5 . 6 8 minutes...
-
Propose a "bargain" that would resolve a dispute from your personal or professional life. Summarize the dispute, propose a "bargain" to resolve the dispute then explain the "net gain" from the...
-
A charged electron is moving through a magnetic field at right angles with velocity 3.2x10 6 m/s. Determine the force if the strength of the magnetic field is 0.70 N.
-
Find the pressure exerted by a force of 72 Newtons on an area of 6 m 3 . Give your answer in N/m
-
Give an example of a cash flow hedge and an example of a fair value hedge.
-
Reread the discussion leading to the result given in (7). Does the matrix sI - A always have an inverse? Discuss.
-
A three-wire system supplies two loads A and B as shown in Fig. 2.125 . Load A consists of a motor drawing a current of 8 A, while load B is a PC drawing 2 A. Assuming 10 h/day of use for 365 days...
-
As a design engineer, you are asked to design a lighting system consisting of a 70-W power supply and two light bulbs as shown in Fig. 2.124 . You must select the two bulbs from the following three...
-
If the three bulbs of Prob. 2.59 are connected in parallel to the 120-V source, calculate the current through each bulb. Prob 2.59 An enterprising young man travels to Europe carrying three light...
-
1. Write the right-hand side equation for the following terms: (a) p(s',rlsa) Pr (???) (b) R(sa) = E [???] (c) (sa) = Pr (???) (d) * = argmax ??? (e) V(s) = ??? (f) Q(s,a) = ???
-
4. At which x-values is the function: sin(3x) x+5 continuous? Express your answer in interval notation.
-
1. The adrenal gland secretes hormones and catecholamines such as epinephrine to regulate the body. The average adult adrenal gland can be estimated as 30 mm in width, 50 mm in length, and up to 10...

Study smarter with the SolutionInn App


