Calculate K p for each reaction. a. NO4(8) = 2 NO(g) b. N(g) + 3 H(g) =
Question:
Calculate Kp for each reaction.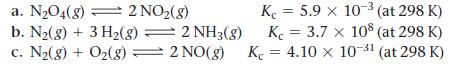
Transcribed Image Text:
a. N₂O4(8) = 2 NO₂(g) b. N₂(g) + 3 H₂(g) = 2 NH3(g) c. N₂(g) + O₂(g) — 2 NO(g) K = 5.9 x 10-³ (at 298 K) K = 3.7 x 108 (at 298 K) K = 4.10 x 10-³1 (at 298 K)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 0% (2 reviews)
a Kp Kc RT An Anmoles of product...View the full answer

Answered By

PALASH JHANWAR
I am a Chartered Accountant with AIR 45 in CA - IPCC. I am a Merit Holder ( B.Com ). The following is my educational details.
PLEASE ACCESS MY RESUME FROM THE FOLLOWING LINK: https://drive.google.com/file/d/1hYR1uch-ff6MRC_cDB07K6VqY9kQ3SFL/view?usp=sharing
3.80+
3+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
This reaction has an equilibrium constant of Kp = 2.2 * 10 6 at 298 K. Calculate Kp for each reaction and predict whether reactants or products will be favored at equilibrium. 2 COF2(g) = CO(g) +...
-
Calculate KP for the following reaction at 25C: H2(g) + I2(g) 2HI(g) G = 2.60 kJ/mol
-
Explain and critically analyze how communication could be utilized to enhance work performance and employee outcomes.
-
5. Vehicle crumple zones are designed to absorb energy during an impact by deforming to reduce transfer of energy to occupants. How much kinetic energy, in Btu, must a crumple zone absorb to fully...
-
A hospital is considering the possibility of two new purchases: new X-ray equipment and new biopsy equipment. Each project would require an investment of $750,000. The expected life for each is five...
-
Compare the performance of C-SCAN and SCAN scheduling, assuming a uniform distribution of requests. Consider the average response time (the time between the arrival of a request and the completion of...
-
What are Lean SE Principles intended to accomplish?
-
Chen, CPA, is the auditor for Greenleaf Manufacturing Corporation, a privately owned company that has a June 30 fiscal year. Greenleaf arranged for a substantial bank loan that was dependent on the...
-
If (log 1068)/(log108) = logganswer, then what is the answer?
-
Write an equilibrium expression for each chemical equation involving one or more solid or liquid reactants or products. a. CO3- (aq) + HO(1) b. 2 KCIO3(s) = 2 KCl(s) + 3 O(g) c. HF(aq) + HO(1) H3O+...
-
Calculate K c for each reaction. a. [(g) = 21(g) Kp 6.26 x 10-22 (at 298 K) b. CH4(g) + HO(g) = CO(g) + 3 H(g) c. I(g) + Cl(g) = 2 ICI(g) = Kp = 7.7 x 1024 (at 298 K) Kp = 81.9 (at 298 K) P
-
Record the current price of the stock for each company you selected in Week 3's Stock Journal. Using MS Excel spreadsheet or MS Word document put stock prices side-by-side, to show your comparison....
-
7. If we assume labor is the only input to production that can be varied, the relationship between the number of barrels of beer Q (in millions) produced at the Boston Beer Company and the number of...
-
Eutopia and the United States of Pollutia(USP)are both developed countries and both members of the WTO. Eutopia is at the forefront of global efforts to combat climate change. It is also a party to...
-
Osborn Manufacturing uses a predetermined overhead rate of $18.60 per direct labor-hour. This predetermined rate was based on a cost formula that estimates $230,640 of total manufacturing overhead...
-
The MPL in the coffee industry in China is 5 and in the rice industry is 10. The MPL in the coffee industry in Italy is 3 and in the rice industry is 9. China has 1,000 workers while Italy has 1,500...
-
On January 1, 2025, Harrington Company has the following defined benefit pension plan balances. Projected benefit obligation Fair value of plan assets Other data related to the pension plan are as...
-
Evaluate the following statement from an analysis viewpoint: A parent company is not responsible for the liabilities of its subsidiaries nor does it own the assets of its subsidiaries. As such,...
-
In a certain school district, 3% of the faculty use none of their sick days in a school year. Find the probability that 5 faculty members selected at random used no sick days in a given year.
-
Determine the current flowing through an element if the charge flow is given by (a) q(t) = (3) mC (b) q(t) = (4t 2 + 20t 4) C (c) q(t) = (15e 3t 2e 18t ) nC (d) q(t) = 5t 2 (3t 3 + 4) pC (e) q(t) =...
-
How much charge is represented by these number of electrons? (a) 6.482 10 17 (b) 1.24 10 18 (c) 2.46 10 19 (d) 1.628 10 20
-
The charge entering the positive terminal of an element is q = 5 sin 4 t mC while the voltage across the element (plus to minus) is v = 3 cos 4 t V (a) Find the power delivered to the element at t...
-
6. (3 pts) Show the d(*) and T(*) values that result from running BSF (bread-first search) algorithm on the graph shown in Fig 3, using vertex a as the starting vertex. (Assuming the adjacency lists...
-
Set up, but do not evaluate, an integral for the volume of the solid obtained by rotating the region bounded by the given curves about the specified line. y = 6x = x, y = x; about x = 7 - 5 ) dx
-
Q5. The following information is given: Pressure head at point A: -0.12m; pressure head at point B: -0.05m; The top soil surface is selected as datum. Determine (1) whether the water flow takes place...

Study smarter with the SolutionInn App


