Consider the gas-phase reaction: The reaction was experimentally determined to be first order in H 2 and
Question:
Consider the gas-phase reaction:![]()
The reaction was experimentally determined to be first order in H2 and first order in I2. Consider the proposed mechanisms.
Proposed mechanism I:![]()
Proposed mechanism II: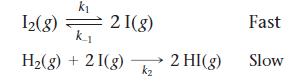
a. Show that both of the proposed mechanisms are valid.
b. What kind of experimental evidence might lead you to favor mechanism II over mechanism I?
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:





