This reaction was experimentally determined to be first order with respect to O 2 and second order
Question:
This reaction was experimentally determined to be first order with respect to O2 and second order with respect to NO:![]()
The diagrams shown here represent reaction mixtures in which the number of each type of molecule represents its relative initial concentration. Which mixture has the fastest initial rate?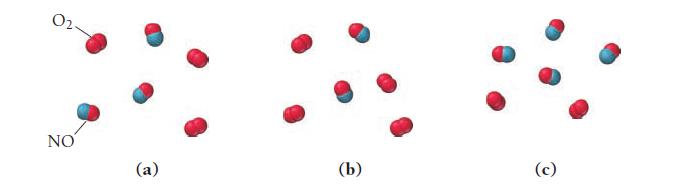
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:





