Consider the reaction: Calculate G rxn for the reaction at 25 C under each of the following
Question:
Consider the reaction:
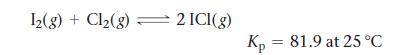 Calculate ΔGrxn for the reaction at 25 °C under each of the following conditions:
Calculate ΔGrxn for the reaction at 25 °C under each of the following conditions:
a. Standard conditions
b. At equilibrium
c. PICl = 2.55 atm; PI2 = 0.325 atm; PCl2 = 0.221 atm
Transcribed Image Text:
12(g) + Cl₂(g) = 2 ICI(g) Kp = 81.9 at 25°C
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (2 reviews)
The standard Gibbs free energy change AG ...View the full answer

Answered By

John Kago
Am a processional practicing accountant with 5 years experience in practice, I also happens to have hands on experience in economic analysis and statistical research for 3 years. am well conversant with Accounting packages, sage, pastel, quick books, hansa world, etc, I have real work experience with Strata, and SPSS
4.70+
31+ Reviews
77+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Consider the reaction: Calculate G rxn for the reaction at 25 C under each of the following conditions: a. Standard conditions b. At equilibrium c. P CH3OH = 1.0 atm; P CO = P H2 = 0.010 atm CO(g) +...
-
Ray Holt Corporation has retained you as a consultant on accounting policies and procedures. During 2019, the company engaged in a number of treasury stock transactions, having foreseen an...
-
The following additional information is available for the Dr. Ivan and Irene Incisor family from Chapters 1-5. Ivan's grandfather died and left a portfolio of municipal bonds. In 2012, they pay Ivan...
-
What problems may be encountered in making a comparative study of remuneration reports?
-
What is the difference between an activity flexible budget and a functional-based (traditional) flexible budget?
-
Refer to the facts described inBE 5-15. Show the DuPont framework's calculation of the three components of the 2011 return on shareholders' equity for Anderson TV and Appliance. In BE 5-15, the 2011...
-
What must be included in a motion for summary judgment?
-
The Byrd Company had the following transactions during 2007 and 2008: 1. On December 24, 2007 a computer was purchased on account from Computers International for $60,000. Terms of the sale were...
-
A delegate to the 1837 Pennsylvania convention remarked that the political community was based on white persons. In this age of expanding political participation, analyze how and why some segments of...
-
Explain the difference between macrostates (external arrangements of particles) and microstates (internal arrangements of particles).
-
Estimate the value of the equilibrium constant at 525 K for each reaction in Problem 73. Problem 73 Use data from Appendix IIB to calculate the equilibrium constants at 25 C for each reaction....
-
Open water oil spills can wreak terrible consequences on the environment and be expensive to clean up. Many physical and biological methods have been developed to recover oil from water surfaces. The...
-
At the beginning of April, Owl Corporation has a balance of $13,000 in the Retained Earnings account. During the month of April, Owl had the following external transactions. Required: Using the...
-
Suppose the commercial banks have a desired reserve ratio of 10%. The entire commercial banking sector holds current demand deposits of $400,000, loans of $300,000 and reserves of $100,000. Explain...
-
Read each of the following cases and view related videos Answer related questions Explain your answers. Copy and paste will receive zero points Be specific. General answers such as "the law violated...
-
what is the difference between commercial banking versus investment banking?
-
How is the civil engineering industry adapting to address the challenges posed by climate change, and what strategies are being implemented to ensure infrastructure resilience in the face of extreme...
-
Explain how accounting information could help you assess whether you could afford to borrow money.
-
A police officer pulls you over and asks to search your vehicle because he suspects you have illegal drugs inside your car. Since he doesn't have reasonable suspicion to search your car, legally he...
-
Name a type of compressor often used for pneumatic fluid power systems.
-
The intake duct to a fan consists of intake louvers, 5.8 m of square duct (800 800 mm), a sudden contraction to a 400-mm-diameter round duct, and 9.25 m of the round duct. Estimate the pressure at...
-
The electric field of an electromagnetic plane wave is given in SI units by (a) What is the wave?s angular frequency? (b) Write an expression for vector k. (c) What is the value of k? (d) Determine...
-
h(x) Find the derivative of the function. 5 133-131/3+8x h'(x) = X Need Help? Read It Watch it
-
How would I work out these two problems, any formulas I need to know? G M Finish update T-Shirt Profit Two fraternities, Sig Ep and Ep Sig, plan to raise money jointly to benefit homeless people on...
-
Provide background information on international (or multinational) firms.

Study smarter with the SolutionInn App


