Copper has two naturally occurring isotopes with masses 62.94 amu and 64.93 amu and has an atomic
Question:
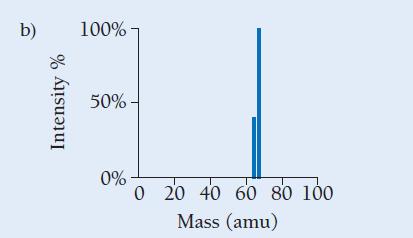
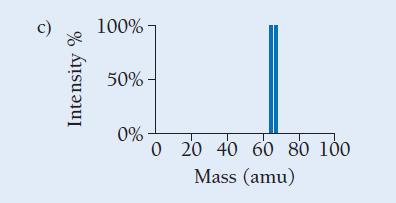
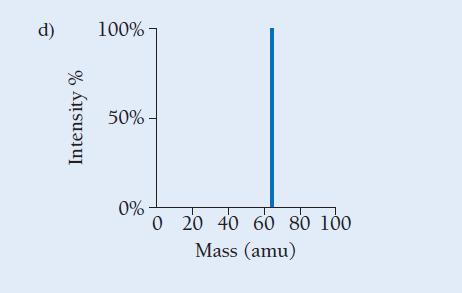
Copper has two naturally occurring isotopes with masses 62.94 amu and 64.93 amu and has an atomic mass of 63.55 amu. Which mass spectrum (of those shown at right) is most likely to correspond to a naturally occurring sample of copper?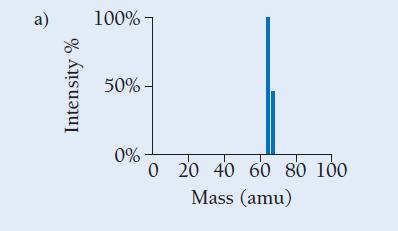
Transcribed Image Text:
a) Intensity % 100% 50% 0% 0 20 40 60 80 100 Mass (amu)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (3 reviews)
a Intensi...View the full answer

Answered By

Surojit Das
I have vast knowledge in the field of Mathematics, Business Management and Marketing. Besides, I have been teaching on the topics Management leadership, Business Administration, Human Resource Management, Business Communication, Accounting, Auditing, Organizer Behaviours, Business Writing, Essay Writing, Copy Writing, Blog Writing since 2020. It is my personality to act quickly in any emergency situations when students need my services. I am very professional and serious in every questions students asked me at the time of dealing any projects. I have been serving detailed, quality, properly analysed research paper through the years.
4.80+
91+ Reviews
278+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
An element has two naturally occurring isotopes with the following masses and abundances: Isotopic Mass (amu) Fractional Abundance 49.9472. 2.500 103 50.9440. 0.9975 What is the atomic mass of this...
-
(A) The masses and percent isotopic abundances of the three naturally occurring isotopes of silicon are 28 Si, 27.9769265325 u, 92.223%; 29 Si, 28.976494700 u, 4.685%; 30 Si, 29.973377017 u, 3.092%....
-
Gallium (Ga) consists of two naturally occurring isotopes with masses of 68.926 and 70.925 amu. (a) How many protons and neutrons are in the nucleus of each isotope? Write the complete atomic symbol...
-
Steve and Linda Hom live in Bartlesville, Oklahoma. Two years ago, they visited Thailand. Linda, a professional chef, was impressed with the cooking methods and the spices used in the Thai food....
-
Star City is considering an investment in the community center that is expected to return the following cash flows: Year Net Cash Flow 1 . . . . . . . . . . . . $ 20,000 2 . . . . . . . . . . . ....
-
In a combustion process, gasoline particles are to be dropped in air at \(200^{\circ} \mathrm{F}\). The particles must drop at least \(10 \mathrm{in}\). in \(1 \mathrm{~s}\). Find the diameter \(d\)...
-
Why is it necessary to create time-phased budgets in projects? What are their major strengths?
-
Identify one or more control procedures (either general or application controls, or both) that would guard against each of the following errors or problems. a. Leslie Thomas, a secretary at the...
-
(Compound annuity) You plan on buying some property in Florida 11 years from today. To do this, you estimate that you will need $45,000 at that time for the purchase. You would like to accumulate...
-
Consider Table 6.19, from a study of nonmetastatic osteosarcoma. The response is whether the subject achieved a three-year disease-free interval. a. Show that each predictor has a significant effect...
-
Explain Millikans oil drop experiment and how it led to the measurement of the electrons charge. Why is the magnitude of the charge of the electron so important?
-
A thief uses a can of sand to replace a solid gold cylinder that sits on a weight-sensitive, alarmed pedestal. The can of sand and the gold cylinder have exactly the same dimensions (length = 22 and...
-
Let A be an n ( n matrix with integer entries. Prove that A is nonsingular and A-1 has integer entries if and only if det(A) = (1.
-
What asset is used by watsonx.governance to organize all the models attempting to solve a single business problem?
-
Cash =$10,000,Contributed Capital =$35,000 Beginning Retained Earnings =$11,000, Dividends =$8,000 Revenues =$55,000, Accounts Payable =$15,000 Equipment =$40,000, Expenses =$33,000 What is the net...
-
A 22-year-old man presents to the Emergency Department with a painless ulcer affecting his glans penis. He admits to unprotected sexual intercourse with a man some two weeks earlier. There is no...
-
in paragraph 4, page 424. The situation mirrors the way we were once fitter-or, at least, leaner-when everyday life required more physical effort, in everything from shovelling coal into the furnace...
-
A company reported the following transactions. Journalize transactions that should be recorded in a purchases journal. March 4 Sold merchandise costing $ 1 , 2 2 0 to Lee for $ 3 , 1 0 0 on credit,...
-
Refer to Exercise. In addition to Internet use, the numbers of years of education were recorded. a. Compute the coefficient of determination. b. Determine the coefficients of the least squares line....
-
Classify each of the following as direct costs or indirect costs of operating the Pediatrics ward for children at the Cleveland Clinic: a. Wi-Fi covering the entire hospital campus b. Net cost of...
-
Calculate H and S if the temperature of 1.75 moles of Hg(l) is increased from 0.00 o C to 75.0 o C at 1 bar. Over this temperature range, C P,m = (J K -1 mol -1 ) 30.093 4.944 10 -3 T/K.
-
Draw the mechanism for each of the following reactions: a. b. c. NaOMe CI NaOEt. Br
-
In the next chapter, we will learn a method for preparing alkynes (compounds containing C ¡ C triple bonds). In the following reaction, a dihalide (a compound with two halogen atoms) is treated...
-
What methodologies can be utilized to perform a comprehensive analysis of an organization's culture, and how do these cultural dimensions influence organizational effectiveness and innovation ?
-
CASE: You are a consultant specializing in L & D, and you have been retained by one of the hotels of Small Ski Resort, a small, traditional ski resort in German speaking Switzerland that has been...
-
Tamar Company manufactures a single product in two departments: Forming and Assembly. Information for the Forming process for May follows. Direct Materials Conversion Units Percent Percent Complete...

Study smarter with the SolutionInn App


