Draw the Lewis structure (including resonance structures) for methyl azide (CH 3 N 3 ). For each
Question:
Draw the Lewis structure (including resonance structures) for methyl azide (CH3N3). For each resonance structure, assign formal charges to all atoms that have formal charge.
Transcribed Image Text:
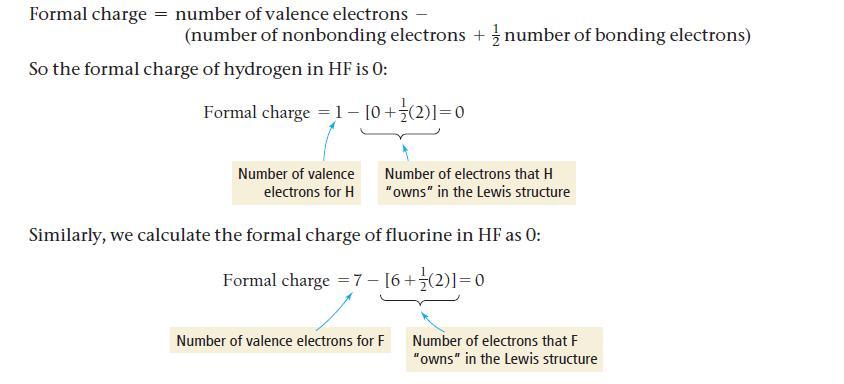
Formal charge number of valence electrons - (number of nonbonding electrons + number of bonding electrons) So the formal charge of hydrogen in HF is 0: Formal charge = 1- [0+ (2)]=0 Number of valence electrons for H Number of electrons that H "owns" in the Lewis structure Similarly, we calculate the formal charge of fluorine in HF as 0: Formal charge = 7 - [6+ (2)] = 0 Number of valence electrons for F Number of electrons that F "owns" in the Lewis structure
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 50% (4 reviews)
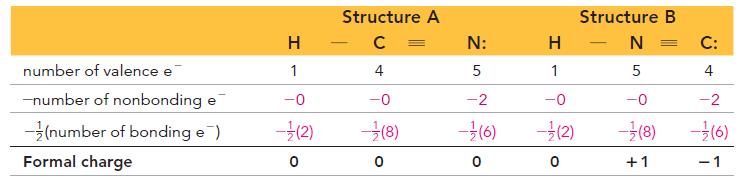
H HCNNN H H HCNNN H I II For structureI Formal charge for all the three Hatom...View the full answer

Answered By

Aysha Ali
my name is ayesha ali. i have done my matriculation in science topics with a+ . then i got admission in the field of computer science and technology in punjab college, lahore. i have passed my final examination of college with a+ also. after that, i got admission in the biggest university of pakistan which is university of the punjab. i am studying business and information technology in my university. i always stand first in my class. i am very brilliant client. my experts always appreciate my work. my projects are very popular in my university because i always complete my work with extreme devotion. i have a great knowledge about all major science topics. science topics always remain my favorite topics. i am also a home expert. i teach many clients at my home ranging from pre-school level to university level. my clients always show excellent result. i am expert in writing essays, reports, speeches, researches and all type of projects. i also have a vast knowledge about business, marketing, cost accounting and finance. i am also expert in making presentations on powerpoint and microsoft word. if you need any sort of help in any topic, please dont hesitate to consult with me. i will provide you the best work at a very reasonable price. i am quality oriented and i have 5 year experience in the following field.
matriculation in science topics; inter in computer science; bachelors in business and information technology
_embed src=http://www.clocklink.com/clocks/0018-orange.swf?timezone=usa_albany& width=200 height=200 wmode=transparent type=application/x-shockwave-flash_
4.40+
11+ Reviews
14+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Draw the Lewis structure (including resonance structures) for the acetate ion (CH 3 COO ). For each resonance structure, assign formal charges to all atoms that have formal charge. Formal charge =...
-
Draw the Lewis structure (including resonance structures) formethyl azide (CH3N3). For each resonance structure, assign formalcharges to all atoms that have formal charge. Draw the Lewis dotstructure...
-
Draw the Lewis structure (including resonance structures) for nitromethane (CH 3 NO 2 ). For each resonance structure, assign formal charges to all atoms that have formal charge.
-
Problem 3: Dorado and Maya are the children of Jess and Sabel. In November 2000 Sabel died intestate leaving P2,000,000 estate before P1,000,000 deductions and P15,000 estate tax. How would the...
-
What are data warehouses? How are they like databases? How do they differ from databases?
-
The Riteway Ad Agency provides cars for its sales staff. In the past, the company has always purchased its cars from a dealer and then sold the cars after three years of use. The companys present...
-
Hemlock Semiconductor Operations, LLC, and SolarWorld Industries Sachsen GmbH, are both companies involved in the manufacture of components for solar power products. Prior to the lawsuit, the two...
-
1. Health information management is a rapidly-growing sector that directly affects health care costs. Every specialized area has its own vocabulary, and health information management is no exception....
-
Explain how the relative prices of rugs and robots in autarky compare with the relative prices when Canada and India start to trade? In your answer explain which country will export/import which...
-
What are the formal charges of the atoms shown in red? CH3 CH3N0: -N- CH3
-
How important is the resonance structure shown here to the overall structure of carbon dioxide? Explain. :0=C:
-
What is the major long-term regulatory reform that the U.S. Department of the Treasury has proposed?
-
What is the difference between the short run and the long run in macroeconomic analysis? Why do macroeconomists differentiate between the two time horizons?
-
In the per-worker production function, what factors determine the level of output per worker? Which one of these factors does the Solow growth model consider to be exogenous?
-
How do you participate in the debate on state ownership versus private ownership?
-
As a manager, is it ethical to threaten your suppliers? Your buyers?
-
Beginning from a steady state in the Solow growth model, explain how an increase in the saving rate will affect the levels and growth rates of capital and output per worker.
-
Look through the costs and benefits that we identified from the completion of the internal market. Do the same costs and benefits arise from the enlarged EU of 27 members?
-
When the concentration of a strong acid is not substantially higher than 1.0 10-7 M, the ionization of water must be taken into account in the calculation of the solution's pH. (a) Derive an...
-
For the manometer shown in Fig. 3.30, calculate (p A - p B ). Water IB 150 mm - Mercury (sg = 13.54) 900 mm Oil (sg = 0.86) 600 mm
-
For the compound manometer shown in Fig. 3.31, calculate the pressure at point A. Oil (sg = 0.90) Water 125 mm 475 mm 250 mm 50 mm Mercury (sg = 13.54)
-
For the compound differential manometer in Fig. 3.32, calculate (p A - p B ). Water, Oil (sg = 0.90) 6 in 8 in 6 in 10 in 6 in Mercury (sg = 13.54)
-
What are the characteristic is NOT typically considered in the selection of market comparator firms
-
Mr. Hernandez controls proxies for 40,000 of the 75,000 outstanding shares of Northern Airlines. Mr. Lueng heads a dissident group that controls the remaining 35,000 shares. There are seven board...
-
1. Why education is considered the bulwark of democracy? 2. What are some of the problems of our educational system? 3. Is adult education necessary in our country? 4. What makes a Schools prestige?...

Study smarter with the SolutionInn App


