Draw the Lewis structure (including resonance structures) for the acetate ion (CH 3 COO ). For
Question:
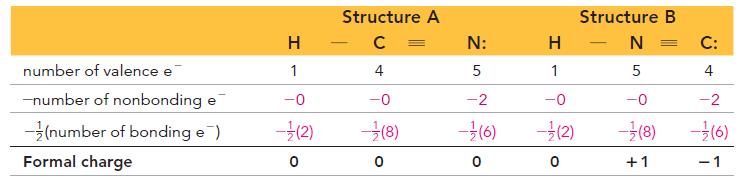
Draw the Lewis structure (including resonance structures) for the acetate ion (CH3COO–). For each resonance structure, assign formal charges to all atoms that have formal charge.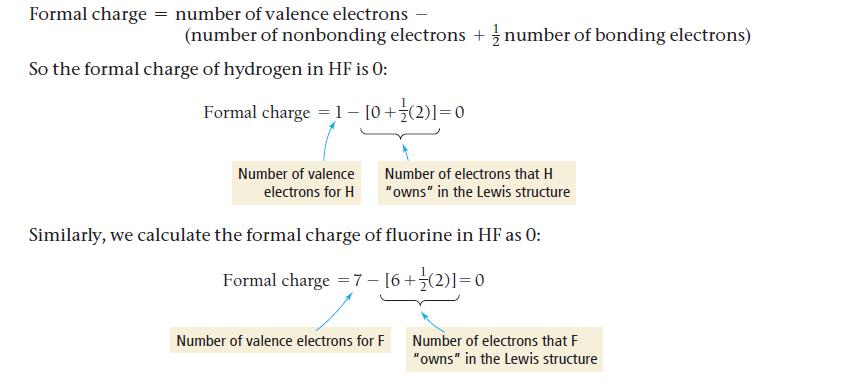
Transcribed Image Text:
Formal charge = number of valence electrons - (number of nonbonding electrons+ number of bonding electrons) So the formal charge of hydrogen in HF is 0: Formal charge = 1- [0+ (2)]=0 Number of valence electrons for H Number of electrons that H "owns" in the Lewis structure Similarly, we calculate the formal charge of fluorine in HF as 0: Formal charge = 7 - [6+ (2)]=0 Number of valence electrons for F Number of electrons that F "owns" in the Lewis structure
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 33% (3 reviews)
H HC...View the full answer

Answered By

Muhammad Umair
I have done job as Embedded System Engineer for just four months but after it i have decided to open my own lab and to work on projects that i can launch my own product in market. I work on different softwares like Proteus, Mikroc to program Embedded Systems. My basic work is on Embedded Systems. I have skills in Autocad, Proteus, C++, C programming and i love to share these skills to other to enhance my knowledge too.
3.50+
1+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Draw the Lewis structure (including resonance structures) for methyl azide (CH 3 N 3 ). For each resonance structure, assign formal charges to all atoms that have formal charge. Formal charge number...
-
Briefly describe some common information system controls that need to be implemented by business managers, not IS professionals.
-
What are the differences between a data entity in structured techniques and an object in object-oriented techniques?
-
5.8 Calculate the U value for the following double- glazed windows assuming the temperatures and the heat transfer coefficients as given in Example 5.1: (a) Ordinary glass with vacuum between the...
-
What are object-oriented databases? What are multimedia databases? How are these two types of databases alike? How are they different?
-
Saxon Products, Inc., is investigating the purchase of a robot for use on the companys assembly line. Selected data relating to the robot are provided below: Engineering studies suggest that use of...
-
In July 2017, Latrice Merritt entered a residential lease with Doran 610 Apartments, LLC. Under the terms of the lease agreement, Merritt was prohibited from installing a private security system in...
-
Shore Company manufactures and sells three products. Relevant per unit data concerning each product are given below. Instructions (a) Compute the contribution margin per unit of the limited resource...
-
Explain briefly why countries trade If two countries, Chile and Agentina start to trade, what will determine what each country will exportor import? Explain why countries do not produce everything...
-
What are the formal charges of the atoms shown in red? CH3 CH3N0: -N- CH3
-
How important is the resonance structure shown here to the overall structure of carbon dioxide? Explain. :0=C:
-
Analysis of operating leverage Rachel Geer has invested in two start-up companies. At the end of the first year, she asks you to evaluate their operating performance. The following operating data...
-
Why is entrepreneurship most often associated with SMEs as opposed to large firms?
-
Why has the FCPA not ended corruption in global business?
-
In the Romer model, how does an increase in total population affect the growth rate of percapita output over time?
-
The U.S. Treasury issues some bonds as Treasury Inflation Indexed Securities, or TIIS, which are bonds adjusted for inflation: hence the yields can be roughly interpreted as real interest rates. Go...
-
How does the institution-based view complement and differ from the industry-based and resource-based views? Why has the institution-based view become a third leg in the strategy tripod?
-
Why is it difficult to estimate the magnitude of the benefits of completing the internal market of the EU?
-
The Alert Company is a closely held investment-services group that has been very successful over the past five years, consistently providing most members of the top management group with 50% bonuses....
-
Figure 3.33 shows a manometer being used to indicate the difference in pressure between two points in a pipe. Calculate (p A - p B ). Oil (sg = 0.90) 3 ft 2'ft 6 ft Water
-
For the well-type manometer in Fig. 3.34, calculate p A . 6,8 in PA Water
-
Figure 3.35 shows an inclined well-type manometer in which the distance L indicates the movement of the gage fluid level as the pressure pA is applied above the well. The gage fluid has a specific...
-
Do you think there should be some international standard for labor rights? If so, what should that standard be and how should it be enforced?
-
Having identified the IHRM challenges presented in the case study, indicate what components you would have considered for inclusion in a training programme for Electrolux and Zanuss prior to, during...
-
Today, Green Branch Coffee's employees began the process of forming a union. The Director of Human Resources wants you to discuss a few important facts about unions so that the coffee shop managers...

Study smarter with the SolutionInn App


