For each of the reactions, calculate the mass (in grams) of the product that forms when 15.39
Question:
For each of the reactions, calculate the mass (in grams) of the product that forms when 15.39 g of the underlined reactant completely reacts. Assume that there is more than enough of the other reactant.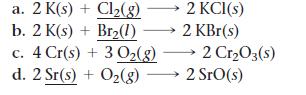
Transcribed Image Text:
a. 2 K(s) + Cl₂(g) b. 2 K(s) + Br₂(1) 2 KCl(s) 2 KBr(s) c. 4 Cr(s) + 30₂(g) → 2 Cr₂O3(s) d. 2 Sr(s) + O₂(g) 2 SrO(s)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 50% (2 reviews)
Heres the calculation for each reaction a 2 Ks Cl2g 2 KCls Molar mass of K 39098 gmol Molar mass of ...View the full answer

Answered By

Nyron Beeput
I am an active educator and professional tutor with substantial experience in Biology and General Science. The past two years I have been tutoring online intensively with high school and college students. I have been teaching for four years and this experience has helped me to hone skills such as patience, dedication and flexibility. I work at the pace of my students and ensure that they understand.
My method of using real life examples that my students can relate to has helped them grasp concepts more readily. I also help students learn how to apply their knowledge and they appreciate that very much.
4.00+
1+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
For each of the reactions, calculate the mass (in grams) of the product that forms when 3.67 g of the underlined reactant completely reacts. Assume that there is more than enough of the other...
-
1. How strong are the competitive forces confronting J. Crew in the market for specialty retail? Do a [Michael Porter] five-forces analysis to support your answer. (see chapter 3 in the textfor...
-
List three specific parts of the Case Guide, Objectives and Strategy Section (See below) that you had the most difficulty understanding. Describe your current understanding of these parts. Provide...
-
Fletcher Fabrication, Inc., produces three products by a joint production process. Raw materials are put into production in Department X, and at the end of processing in this department, three...
-
How did Mr. Sullivans reclassifying some costs as asset purchases affect net income at the time? In the future? How did this action affect cash flows? What does this tell you about the impotence of...
-
Cranberries are harvested by flooding the bogs in which they are grown and raking them into troughs for transport. At the processing plant, the surface moisture on the berries is removed as they roll...
-
Calculate the energy density versus temperature very early in the universe when the temperatures were above \(k T=300 \mathrm{MeV}\). At those temperatures, quarks and gluons were released from...
-
Portland Company's Ironton Plant produces precast ingots for industrial use. Carlos Santiago, who was recently appointed general manager of the Ironton Plant, has just been handed the plant's income...
-
as Suppose X is a normal variable with mean and variance 2. Let f(x) be the density function of X. For a constant 0, define the tilted density function fo(x) = f(x)ex Mo where Me is the normalization...
-
Scenario 2 You are the early childhood teacher in the toddler room. You notice some of your staff do not believe the toddlers are capable of making choices and completing routines on their own. What...
-
Find the limiting reactant for each initial amount of reactants. a. 2 mol Na, 2 mol Br 2 b. 1.8 mol Na, 1.4 mol Br 2 c. 2.5 mol Na, 1 mol Br 2 d. 12.6 mol Na, 6.9 mol Br 2 2 Na(s) + Br(g) Br(g) 2...
-
Sulfuric acid dissolves aluminum metal according to the reaction: Suppose you want to dissolve an aluminum block with a mass of 15.2 g. What minimum mass of H 2 SO 4 (in g) do you need? What mass of...
-
A nuclear plant was established in Hanford, Washington, in 1943. Over the years, a significant amount of strontium 90 and cesium 137 leaked into the Columbia River. In a study to determine how much...
-
Can a defendant file a cross-claim and counterclaim? What would have to be true in terms of the number of parties to the action?
-
Does a court have jurisdiction over a non-resident? Review Burnham v. Superior Court of California , 495 U.S. 604 ( 1990) and explain.
-
Write a complete JavaScript program to prompt the user for 4 grades of tests out of 25 then call a function FindTotal to calculate and display the total The user should enter the inputs in an HTML5...
-
The following information was drawn from the accounting records of Jones Company. Net sales $372,414 Net income 54,000 Average total assets 530,000 Average total liabilities 320,000 Average total...
-
A 100-degree arc of a circle has a length of 7cm. To the nearest centimeter, what is the radius of the circle?
-
The following procedures were recently installed by The Ironworks Shop: a. All sales are rung up on the cash register, and a receipt is given to the customer. All sales are recorded on a record...
-
Explain why each of the following is either a private good or a public good: traffic lights, in line skates, a city park, a chicken salad sandwich, a tennis racket, national defense, a coastal...
-
When ortho-bromonitrobenzene is treated with NaOH at elevated temperature, only one product is formed. (a) Draw the product. (b) Identify the intermediate formed en route to the product. (c) Would...
-
Predict the change in the partial pressure of CO2 as Xe gas is introduced into the reaction vessel at constant volume and temperature. CO(g) + 1/2O 2 (g) CO 2 (g) at equilibrium for which H o R =...
-
You place 3.00 mol of NOCl(g) in a reaction vessel. Equilibrium is established with respect to the decomposition reaction NOCl(g) NO(g) + 1/2Cl 2 (g). a. Derive an expression for K P in terms of the...
-
1. A thin film is laid over a glass pane as shown. White light is incident on the film, coming straight in. At a point where the light is incident on the film, it appears green ( = 525 nm). Find (a)...
-
(20%) The output of an argon ion laser can consist of a number of modes of frequency that match the cavity resonance condition and are within the gain bandwidth of the lasing transition. Assume the...
-
A single nuclear reactor produces 2.6GW of electrical power, and has a generator voltage of 22kV.What percentage of power would be lost from our nuclear reactor in three-line transmission that goes...

Study smarter with the SolutionInn App


