Free radicals are important in many environmentally significant reactions (see the Chemistry in the Environment box on
Question:
Free radicals are important in many environmentally significant reactions (see the Chemistry in the Environment box on free radicals in this chapter). For example, photochemical smog—smog that results from the action of sunlight on air pollutants—forms in part by these two steps: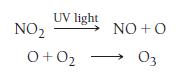
The product of this reaction, ozone, is a pollutant in the lower atmosphere. (Upper atmospheric ozone is a natural part of the atmosphere that protects life on Earth from ultraviolet light.)
Ozone is an eye and lung irritant and also accelerates the weathering of rubber products. Rewrite the given reactions using the Lewis structure of each reactant and product. Identify the free radicals.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:





