Given the data, calculate S vap for each of the first four liquids. (S vap = H
Question:
Given the data, calculate ΔSvap for each of the first four liquids.
(ΔSvap = ΔHvap/T, where T is in K)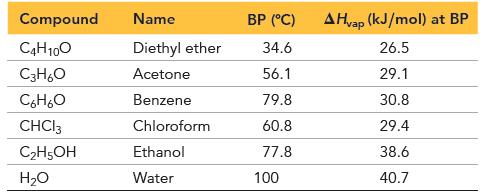
All four values should be close to each other. Predict whether the last two liquids in the table have ΔSvap in this same range. If not, predict whether it is larger or smaller and explain. Verify your prediction.
Transcribed Image Text:
Compound C4H10O C3H6O C6H₂O CHCI 3 C₂H5OH H₂O Name Diethyl ether Acetone Benzene Chloroform Ethanol Water BP (°C) 34.6 56.1 79.8 60.8 77.8 100 AHvap (kJ/mol) at BP 26.5 29.1 30.8 29.4 38.6 40.7
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 50% (2 reviews)
ASvap diethyl ether 861 Jmol K ASvap acetone 884 Jmol K AS...View the full answer

Answered By

Asd fgh
sadasmdna,smdna,smdna,msdn,masdn,masnd,masnd,m asd.as,dmas,dma.,sd as.dmas.,dma.,s ma.,sdm.,as mda.,smd.,asmd.,asmd.,asmd.,asm
5.00+
1+ Reviews
15+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Planning is one of the most important management functions in any business. A front office managers first step in planning should involve determine the departments goals. Planning also includes...
-
Managing Scope Changes Case Study Scope changes on a project can occur regardless of how well the project is planned or executed. Scope changes can be the result of something that was omitted during...
-
The file DVD Movies.xlsx contains a large data set of 10,000 customer transactions for a fictional chain of video stores in the United States. Each row corresponds to a different customer and lists...
-
Why does the cost to load in an unconstrained system is lower compared to contrained with N - 1 Contingency in PLEXOS Modelling? What are the factors that affect the increase in cost to load during...
-
Refer to the information in C1. Jordan Smith, the president of Eagle Manufacturing, wants to improve the quality of the companys operations and products. She believes waste exists in the design and...
-
What is the role of the balance sheet in the business plan?
-
Using the data in question 4, Department Xs contribution to overhead as a percentage of sales is a. 20%. c. 12%. e. 32%. b. 30%. d. 48%. Data From Question 4 A company operates three retail...
-
Polk Company builds custom fishing lures for sporting goods stores. In its first year of operations, 2012, the company incurred the following costs. Variable Cost per Unit Direct materials...
-
1. A ray of light enters glass (index 1.570) from air at an incident angle of 25. Find the angles of refraction and of deviation. 2. A light ray is directed through air (index 1.000) at a 25 angle of...
-
The salt ammonium nitrate can follow three modes of decomposition: (a) To HNO 3 (g) and NH 3 (g), (b) To N 2 O(g) and H 2 O(g), and (c) To N 2 (g), O 2 (g), and H 2 O(g). Calculate G rxn for each...
-
Calculate the entropy of each state and rank the states in order of increasing entropy. (a) (b) (c)
-
An automobile club that pays for emergency road services (ERS) requested by its members wishes to estimate the proportions of the different types of ERS requests. Upon examining a sample of 2927 ERS...
-
Overview Dynamic pricing is a collection of pricing strategies used by firms and organizations to enhance profits. You will begin by exploring pricing techniques that operate in the market in real...
-
? ? The task is to create a NoSQL database for a library to manage its book records. Task 1 . 1 1 . 1 Write a python program to: Create a MongoDB database named library and a new collection named...
-
a. Write the binary string for the message "hello friends". How many bits are needed for Huffiman code? How many bits are needed for ASCII code? b. Decode the following binary string using the above...
-
You have configured LDAP over SSL (LDAPS) with default settings to secure directory service queries across subnets. Which port must be open on the subnet firewall?
-
We have engaged in several excellent discussions over the semester. For this discussion, post a farewell to your colleagues and offer them bit of knowledge, advice, even a joke. Then check out a few...
-
The following is a full-blown demand equation for Pizza QD = - 100P + 1.5Phd - 5Psd + 20A + 15Pop P = Price of pizza Phd = Price of hot dogs ($1.00, use 100 cents) Psd = Price of soft drink ($0.75,...
-
Which of the ocean zones shown would be home to each of the following organisms: lobster, coral, mussel, porpoise, and dragonfish? For those organisms you identify as living in the pelagic...
-
A child plays on a bungee cord and oscillates with a certain frequency f. An adult with a mass that is five times greater than that of the child then uses the same bungee cord. What is the ratio of...
-
Suppose Figure P11.3 describes the displacement of a masson- a-spring harmonic oscillator.? Figure P11.3 ? (a) If the mass is m = 2.0 kg, what is the spring constant?? (b) Estimate the velocity at t...
-
A mass m = 4.5 kg is attached to a vertical spring with k = 200 N/m and is set into motion. (a) What is the frequency of the oscillation? (b) If the amplitude of the oscillation is 3.5 cm, what is...
-
Flag question Rick & Morty conduct business as partners operating a toy store. Their partnership agreement specifies that all profits and losses are to be distributed equally after partners salaries....
-
GEE Limited need to raise finance to expand its product range. In the last board meeting, it was agreed that the additional Kenya Shilling 40 million be raised as follows: i. 100,000 new ordinary...
-
3. (10 points) Assume the following information: 1-year deposit rate offered on U.S. dollars 1-year deposit rate offered on Singapore dollars 1-year forward rate of Singapore dollars Spot rate of...

Study smarter with the SolutionInn App


