Is a spontaneous redox reaction obtained by pairing any reduction half-reaction with one listed above it or
Question:
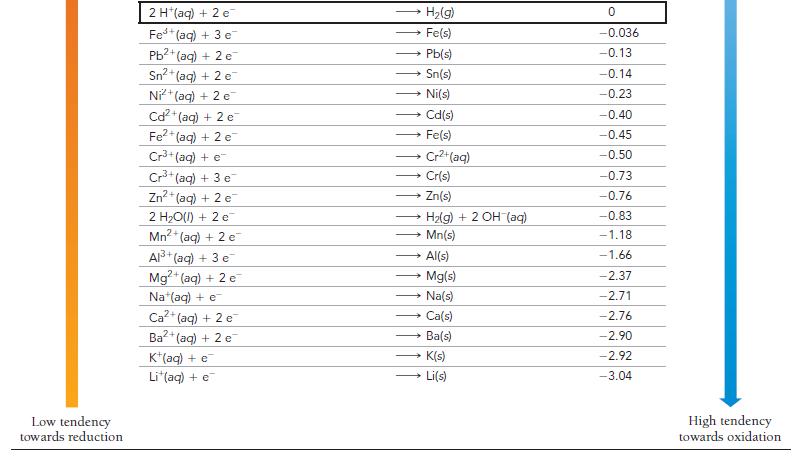
Is a spontaneous redox reaction obtained by pairing any reduction half-reaction with one listed above it or with one listed below it in Table 20.1?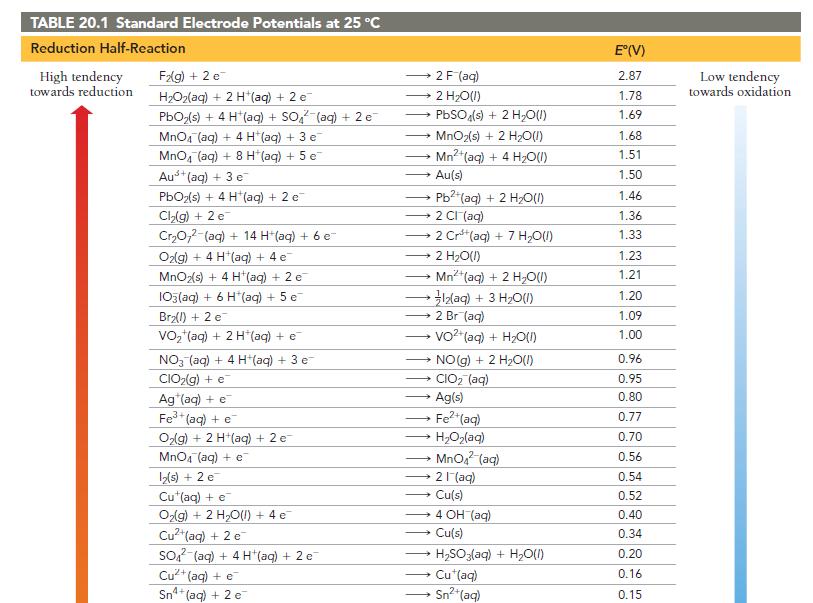
Transcribed Image Text:
TABLE 20.1 Standard Electrode Potentials at 25 °C Reduction Half-Reaction High tendency towards reduction F₂(g) + 2 e H₂O₂(aq) + 2 H+ (aq) + 2 e PbO₂(s) + 4 H (aq) + SO₂ (aq) + 2 e MnO4 (aq) + 4 H*(aq) + 3 e MnO₂ (aq) + 8 H+ (aq) + 5 e Aus+ (aq) + 3 e PbO₂(s) + 4 H*(aq) + 2 e Cl₂(g) + 2 c Cr₂O72-(aq) + 14 H+ (aq) + 6 e O2(g) + 4 H(aq) + 4 e MnO₂(s) + 4 H*(aq) + 2 e 103(aq) + 6 H*(aq) + 5 e Br₂(l) + 2 e VO₂ (aq) + 2 H+ (aq) + e NO3(aq) + 4 H+ (aq) + 3 e CIO₂(g) + e Ag (aq) + e Fe³+ (aq) + e O₂(g) + 2 H+(aq) + 2 c MnO4 (aq) + e 12(s) + 2 e Cu (aq) + e O₂(g) + 2 H₂O(l) + 4 e Cu²+ (aq) + 2 e SO² (aq) + 4 H(aq) + 2 e Cu²+ (aq) + e Sn4+ (aq) + 2 e 2 F (aq) → 2 H₂O(l) → → 2 CI (aq) PbSO4(s) + 2 H₂O(l) MnO₂(s) + 2 H₂O(1) Mn²+ (aq) + 4H₂O(1) Au(s) → 2 H₂O(l) → Pb2+ (aq) + 2 H₂O(l) — Mnt(aq) + 2 H,O(1) * 3lz(aq) + 3 H2O(I) →→→2 Br (aq) 2 Cr³+ (aq) + 7 H₂O(l) → → NO(g) + 2 H₂O(l) CIO₂ (aq) → Ag(s) VO²+ (aq) + H₂O(1) → - → HyOz(aq) Fe²+ (aq) → Cu(s) MnO₂ (aq) 21 (aq) →→→ 4 OH (aq) → Cu(s) H₂SO3(aq) + H₂O(l) Cu (aq) Sn²+ (aq) E°(V) 2.87 1.78 1.69 1.68 1.51 1.50 1.46 1.36 1.33 1.23 1.21 1.20 1.09 1.00 0.96 0.95 0.80 0.77 0.70 0.56 0.54 0.52 0.40 0.34 0.20 0.16 0.15 Low tendency towards oxidation
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (1 review)
Look for pairings where the reduction potential of the halfreaction to be reduced is greater more po...View the full answer

Answered By

Lilian Nyambura
Hi, am Lilian Nyambura, With extensive experience in the writing industry, I am the best fit for your writing projects. I am currently pursuing a B.A. in Business Administration. With over 5 years of experience, I can comfortably say I am good in article writing, editing and proofreading, academic writing, resumes and cover letters. I have good command over English grammar, English Basic Skills, English Spelling, English Vocabulary, U.S. English Sentence Structure, U.K. or U.S. English Punctuation and other grammar related topics. Let me help you with all your essays, assignments, projects, dissertations, online exams and other related tasks. Quality is my goal.
4.80+
378+ Reviews
750+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
The following additional information is available for the Dr. Ivan and Irene Incisor family from Chapters 1-6. On December 12, Irene purchased the building where her store is located. She paid...
-
Carol Harris, Ph.D, CPA, is a single taxpayer and she lives at 674 Yankee Street, Durham, NC 27409. Her Social Security number is 793-52-4335. Carol is an Associate Professor of Accounting at a local...
-
Case Study: Quick Fix Dental Practice Technology requirements Application must be built using Visual Studio 2019 or Visual Studio 2017, professional or enterprise. The community edition is not...
-
For the car suspension shown in Figure determine the differential equation, transfer function and state space model. Plot the position of the car and the wheel after the car hits a unit bump (that...
-
Gilbert, Inc., which uses a process costing system, makes a chemical used as a food preservative. The manufacturing process involves Departments A and B. the company had the following total costs and...
-
CRST Van Expedited, Inc. (CRST), a trucking company, sponsors a driver training program to help its new hires become certified truck drivers. At a certain point in the training program, the driver...
-
Explain how the control variate technique is implemented.
-
The following selected transactions were completed by Amsterdam Supply Co., which sells office supplies primarily to wholesalers and occasionally to retail customers. Also note that the company uses...
-
Activity 1 Response: Measure the temperature of the environment (ambient temperature), the human body (both forehead and under the tongue), and two other objects in the room. Record this information...
-
Which reaction occurs at the cathode of an electrolytic cell containing a mixture of molten KCl and ZnCl 2 ? a) K(s)K+ (1) + e b) K (1) + e K(s) c) Zn+(1) + 2e Zn(s) d) 2 CI (1) Cl(g) + 2 e-
-
Describe the standard hydrogen electrode (SHE) and explain its use in determining standard electrode potentials.
-
What are the effects of wear flat on the grinding operation? Are there similarities to the effects of flank wear in metal cutting? Explain.
-
Prepare an updated balance sheet for July 31, based on receipts and expenditure Notes: Wages Payable:1 employee; 6 months remaining, at $1,250 /month Lease Payable: 6 months remaining on current...
-
Low Nail Co. now reports the following data: Annual demand increased to 2,200 kegs per year Inventory carrying costs has increased to $1.52 per keg Order processing costs are now only $52.00 per...
-
During 20xx, the total net amount paid for salaries and wages amounted to P5,000,000 after the following deductions: SSS employees contribution 425,500 Pag-Ibig employees contribution 130,300...
-
Clyde is enrolled in Medicare Parts A and B. He had a knee arthroscopy in January. This is Clyde's first health encounter for the year so he has not met his deductible. The approved amount for his...
-
Veronica and Manny Dunn, married taxpayers, obtained a mortgage in the amount of $550,000 in January of 2023 when they purchased their house in Chatsworth. In December of the same year, Veronica and...
-
A. Explain the concept of the multiplier, and explain the role of the marginal propensity to consume in determining the size of the multiplier. B. Explain how the size of the multiplier will change...
-
Sundial Technologies produces and sells customized network systems in New Brunswick. The company offers a 60-day, all software and labor-and an extra 90-day, parts-only- warranty on all of its...
-
(a) Water flows out of a shower in a typical house at a rate of 20 liters/min. If this shower is fed through a pipe that is 2.0 cm in diameter and 15 m from the high-pressure water tank, what is the...
-
Collapsing submarines. The collapse depth of a typical military submarinethat is, the depth at which the submarine would be crushed by the force due to water pressureis 700 m. If the pressure on the...
-
Measuring air speed. An airplane pilot must always know her speed relative to the surrounding air. Air speed that is too low can cause the plane to stall, whereas air speed that is too high can cause...
-
Explain the sources of South African law. Discuss the position of the Companies Act in relation to the two types of recognized companies. Companies are classified into two categories, Profit and Not...
-
Jamie is a financial manager working for ChemEx, a New Zealand manufacturer of chemicals. Jamie is doing some analysis on the company's cost of capital and has collated the information below. Stock...
-
Discuss the motivational initiatives the Company should adopt to ease the transition after retrenchments.

Study smarter with the SolutionInn App


