Most of the second row transition metals do not follow the normal orbital filling pattern. Five of
Question:
Most of the second row transition metals do not follow the normal orbital filling pattern. Five of them—Nb, Mo, Ru, Rh, and Ag—have a [Kr] 5s14dx configuration and Pd has a [Kr] 4d10 configuration.
Write the ground state electron configuration for each species.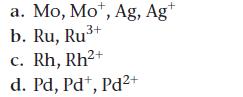
Transcribed Image Text:
a. Mo, Mot, Ag, Ag+ 3+ b. Ru, Ru³+ 2+ c. Rh, Rh²+ d. Pd, Pdt, Pd²+
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (3 reviews)
Based on the information provided here are the ground state electron configurations for each species ...View the full answer

Answered By

Deborah Joseph
My experience has a tutor has helped me with learning and relearning. You learn everyday actually and there are changes that are made to the curriculum every time so being a tutor has helped in keeping me updated about the present curriculum and all.
I have also been able to help over 100 students achieve better grades particularly in the categories of Math and Biology both in their internal examinations and external examinations.
5.00+
2+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
The two most common isotopes of uranium are 235U and 238U. (a) Compare the number of protons, the number of electrons, and the number of neutrons in atoms of these two isotopes. (b) Using the...
-
Write the ground state electron configurations for each of the following atoms: (a) Boron (b), (b) Calcium (Ca), (c) Zinc (Zn), and (d) tin (Sn).
-
Refer to the periodic table to write the electron configuration for selenium (Se). Orbital Blocks of the Periodic Table Periods 1 2 3 4 6 Groups 1 1A 7 4x523=~=- 2 1s 2A 25 Na 37 5 Rb 5s 12 Mg 3s 35...
-
Answer the following question based on the information presented for Cloud 9 in the appendix to this text and the current and earlier chapters. You should also consider your answers to the case study...
-
Accounting firms are among the worlds largest partnerships and provide a wide range of attractive careers for business and accounting majors. Through the needles website at...
-
What are the seven wastes? Can you discuss them in terms of a business you are familiar with?
-
What is the plain-meaning rule?
-
The cost of equipment purchased by Charleston, Inc., on June 1, 2012, is $89,000. It is estimated that the machine will have a $5,000 salvage value at the end of its service life. Its service life is...
-
Ricardo works for Bank B and is talking about loans with a consumer. He provides the consumer with a general explanation regarding the basic qualifications of a loan. Although the consumer plans to...
-
Draw the Lewis diagrams for each ligand. Indicate the lone pair(s) that may be donated to the metal. Indicate any you expect to be bidentate or polydentate. a. CN b. Bipyridine (bipy), which has the...
-
Recall that Cr and Cu are exceptions to the normal orbital filling, resulting in a [Ar] 4s 1 3d x configuration. Write the ground state electron configuration for each species. Cr, Cr, Cr2+, Cr+ a....
-
Two fair dice are rolled. Find the probabilities in parts (a)(d). (a) The sum of the dots is at least 10. (b) The sum of the dots is less than 10. (c) The sum of the dots is either 7 or at least 10....
-
What are three things you learned about in text APA citing. Use full sentences - you should have at least 5 sentences
-
How will you communicate? What method will you use? What communication style will you adopt? Why is this style suitable? What communication challenges/risks do you anticipate (at...
-
a member of a project team in your company working on critical organizational issues, choose one (1) topic out of the 3 selections below and write a 3-minute speech about your chosen topic: 1. How...
-
Ann opened an Office Cleaning Service company on January 1, 2020. During 2020 she had the following transactions. (1) She started the business with investing $30,000 of her own money (business was...
-
What is synthesis on multiculturism in workplaces.? how multiculturism affect in good and bad ways in workplaces.?
-
Computer technology in accounting systems and electronic transfer of funds has changed since the 1980s. Have these changes reduced or increased the likelihood of billing scheme frauds?
-
(a) Find the equation of the tangent line to f(x) = x 3 at the point where x = 2. (b) Graph the tangent line and the function on the same axes. If the tangent line is used to estimate values of the...
-
A constant electric field generally produces a constant drift velocity. How is this consistent with Newtons assertion that force results in acceleration, not velocity?
-
Explain the difference between the current and current density.
-
A parallel-plate capacitor is connected to a battery that imposes a potential difference V between its plates. If a dielectric slab is inserted between the plates, what happens to (a) the potential...
-
Generally, when work orders evidencing [a] are summarized on RUS Form 219, Inventory of Work Orders, the amount shown in Column 9, Loan Funds Subject to Advance by RUS, is equal to the excess of the...
-
If Melanie wants to finance a new home for $320,000 with 4%,30 year mortgage or a 3.5%, 15 year mortgage. What is the monthly payment on each one of the above alternatives?
-
An ordinary annuity assumption means that cash is expected to change hands Multiple Choice in a series of exchanges made at the beginning of each period. in a series of exchanges made at the end of...

Study smarter with the SolutionInn App


