Pick an appropriate solvent from Table 14.3 to dissolve each substance. State the kind of intermolecular forces
Question:
Pick an appropriate solvent from Table 14.3 to dissolve each substance. State the kind of intermolecular forces that would occur between the solute and solvent in each case.
a. Motor oil (nonpolar)
b. Ethanol (polar, contains an OH group)
c. Lard (nonpolar)
d. Potassium chloride (ionic)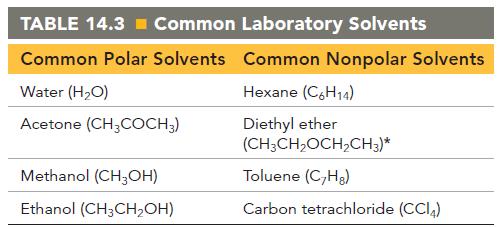
Transcribed Image Text:
TABLE 14.3= Common Laboratory Solvents Common Polar Solvents Common Nonpolar Solvents Hexane (C6H14) Diethyl ether (CH₂CH₂OCH₂CH3)* Toluene (C₂Hg) Carbon tetrachloride (CC14) Water (H₂O) Acetone (CH3COCH3) Methanol (CH3OH) Ethanol (CH3CH₂OH)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 50% (4 reviews)
a Hexane toluene or CCl 4 dispersion for...View the full answer

Answered By

AJIN kuriakose
I have completed B.Tech in Electrical Engineering & Masters in Power & Control From one of the best universities in India. I got the 99.05 percentile in the Gate Electrical Engineering Exam. I can Help students solving assignments in Electrical subjects like Power Electronics, Control system, Analog, Network Theory & Engineering Mathematics. Clear your fundamentals and develop problem-solving skills and analytical skills to crack the exam.
Get guidance and the opportunity to learn from experienced...
I can provide tuition for Electrical engineering subjects (Power Electronics, Digital electronics, Network Theory, Control System & Engineering Mathematics). The toughest subject of Electrical engineering can be made simple in online classes...
I can also solve it.
1 .I can help you with your assignments or exams or quiz or tutoring.
2. Very strict to the deadlines.
Message me for any help in assignments, live sessions. I am here to help students for all assignments, tests and exams and I will make sure you always get _95% In your subject.
Contact me in solution inn for any help in your semester, projects and for many more things . Also feel free to contact me through solution inn and for any advise related to tutoring and how it works here.thank you.
5.00+
5+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Pick an appropriate solvent from Table 14.3 to dissolve each substance. State the kind of intermolecular forces that would occur between the solute and solvent in each case. a. Isopropyl alcohol...
-
A.) Pick an appropriate solvent to dissolve acetic acid (polar,contains an OH group) . Water (H 2 O) Acetone (CH 3 COCH 3 ) Methanol (CH 3 OH) Ethanol (CH 3 CH 2 OH) Hexane (C 6 H 14 ) Diethyl...
-
For each compound, would you expect greater solubility in water or in hexane? Indicate the kinds of intermolecular forces that would occur between the solute and the solvent in which the molecule is...
-
Dwights preferences over beer and whiskey satisfy more is better, but are concave (thus violating the usual assumption of convexity). a. On a diagram, sketch what such indifference curves would look...
-
Hemple produces a variety of pocket PCs. Due to competitive pressures, the company is implementing an activity-based management (ABM) system with the objective of reducing costs. ABM focuses...
-
Normal butane and isobutane have boiling temperatures of 0.5 and 12.3C (31.1 and 9.9F), respectively. Briefly explain this behavior on the basis of their molecular structures, as presented in Section...
-
Define the following: a. Asset b. Liability c. Net asset
-
This problem continues the Draper Consulting, Inc., situation from Problem 2-62 of Chapter 2. Start from the trial balance and the posted T-accounts that Draper Consulting, Inc., prepared at December...
-
10. Label and explain in details the following diagram A 12. 1 Give three functions of E. Zinc E B + C D Copper 13. Give an ideal principle of predicting a performance of a fuel cell and give an...
-
What keeps the particles in a colloidal dispersion from coalescing?
-
What is the Tyndall effect, and how can it be used to help identify colloidal dispersions?
-
The Greek Connection had sales of $32 million and a cost of goods sold of $12.8 million in 2015. A simplified balance sheet for the firm appears below: a. Calculate The Greek Connections net working...
-
Name the two most commonly used overall methods of accounting.
-
What tax benefits are embodied in specific legacies and bequests?
-
What is substantially appreciated inventory?
-
In what situation will a corporate shareholder receive non recognition treatment in a complete liquidation?
-
What are four broad types of like-kind exchanges?
-
Castor, Inc. is preparing its master budget for the quarter ended June 30. Budgeted sales and cash payments for merchandise for the next three months follow: Sales are 50% cash and 50% on credit. All...
-
If the cylinder described in Problem 21.3 were initially heated to 500F, how long would it take for the center of the cylinder to cool to 240F if it were constructed of a. Copper? b. Brass? c. Nickel?
-
A car is moving with a velocity of 20 m/s when the brakes are applied and the wheels lock (stop spinning). The car then slides to a stop in 40 m. Find the coefficient of kinetic friction between the...
-
You are given the job of moving a refrigerator of mass 100 kg across a horizontal floor. The coefficient of static friction between the refrigerator and the floor is 0.45. What is the minimum force...
-
Your moving company runs out of rope and hand trucks, so you are forced to push two crates along the floor as shown in Figure P3.39. The crates are moving at constant velocity, their masses are m 1 -...
-
8. A horse is trapped in a well. Its owner attaches one end of a light, inextensible rope to the horse, and the other end to her tractor. The tractor has a mass of 2100 kg and the horse has a mass of...
-
Proposal Business Report with SWOT Analysis 4-13-2023 I need some help putting all of my research for a final report on Ford Company, with some positive ideas that were given to me, I have included...
-
iv) Is the set of vectors the equation -2 8 }} linearly independent? If not, enter a non-zero solution to 2 0 +B 5 + 5 = 0 3 -8 Otherwise enter the zero solution (i.e. [0,0,0]). [a, ,] = ||

Study smarter with the SolutionInn App


