The decomposition of Br 2 is followed as a function of time; two different plots of the
Question:
The decomposition of Br2 is followed as a function of time; two different plots of the data are shown. Determine the order and rate constant for the reaction.![In [Br] -0.5- -1 -1.5 -2- -2.5+ 0 20 40 Time (s) 60 80](https://dsd5zvtm8ll6.cloudfront.net/images/question_images/1700/1/9/6/5236556f0ab2733a1700196522339.jpg)
![1/[Br] 10. 9. 8- 7 5- 4 3- 2. 1 0 0 20 40 Time (s) 60 80](https://dsd5zvtm8ll6.cloudfront.net/images/question_images/1700/1/9/6/5306556f0b21119e1700196529185.jpg)
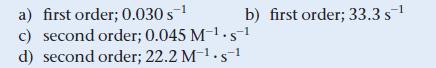
Transcribed Image Text:
In [Br₂] -0.5- -1 -1.5 -2- -2.5+ 0 20 40 Time (s) 60 80
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (4 reviews)
a Fi...View the full answer

Answered By

Madhur Jain
I have 6 years of rich teaching experience in subjects like Mathematics, Accounting, and Entrance Exams preparation. With my experience, I am able to quickly adapt to the student's level of understanding and make the best use of his time.
I focus on teaching concepts along with the applications and what separates me is the connection I create with my students. I am well qualified for working on complex problems and reaching out to the solutions in minimal time. I was also awarded 'The Best Tutor Award' for 2 consecutive years in my previous job.
Hoping to get to work on some really interesting problems here.
5.00+
1+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Determine the average Cp value in KJ/kg-K of a gas if 522 KJ/kg of heat is necessary to raise the temperature from 3OOK to 800K making the pressure constant * 1.038 1.026 1.440 1.044 What is the...
-
Cyclopropane isomerizes into propene when heated to 500C in the gas phase. The extent of conversion for various initial pressures has been followed by gas chromatography by allowing the reaction to...
-
XYZ Co. uses two types of materials in the production. The following standards were developed: Material M1 M2 Yield Material M1 M2 Standard Mix Standard Unit Price 2 units $20 per unit 3 units 12 per...
-
After assembly, a finished TV is left turned on for one full day (24 h) to determine whether the product is reliable. On average, two TVs break down each day. Yesterday 500 TVs were produced. What is...
-
Olgivie Company had a bad year in 2013. For the first time in its history, it operated at a loss. The company's income statement showed the following results from selling 60,000 units of product:...
-
On the basis of the following stockholders?? equity accounts, indicate the items, exclusive of net income, to be reported on the statement of cash flows. There were no unpaid dividends at either the...
-
Copy your worksheet from Question 6 into another worksheet. Change the increase from 10% to 18%. Protect the worksheet, so that changes cannot be made. Question 6 Open a new spreadsheet. Type...
-
Smart Video Company is a manufacturer of videoconferencing products. Maintaining the videoconferencing equipment is an important area of customer satisfaction. A recent downturn in the computer...
-
How can we add database connection script to store form input values into MySQL Database Table such as LogIn tables.
-
Consider a simple reaction in which reactant A forms products: What is the rate law if the reaction is zero order with respect to A? First order? Second order? For each case, explain how a doubling...
-
Explain the difference between the average rate of reaction and the instantaneous rate of reaction.
-
Estimate the volume of the solid that lies above the square R= [0, 2] [0, 2] and below the elliptic paraboloid z = 16 x 2 2y 2 . Divide R into four equal squares and choose the sample point to be...
-
An application for insurance is not part of the insurance contract. (True/False)
-
If an insurance company does not act in good faith, what can the insured do?
-
How can a bailment occur if the bailor does not voluntarily deliver the property to the bailee?
-
The government can take private property for private uses only. (True/False)
-
An antilapse clause provides that an insurance policy lapses if the insured does not pay a premium exactly on time. (True/False)
-
Milport Co. pays salaries monthly on the last day of the month. The following information is available from Milport Co. for the month ended December 31, 2013. Administrative salaries ........ $85,000...
-
Read Case Study Google: Dont Be Evil Unless and answer the following: Given its mission of providing information to the world, should Google censor searches in China?
-
A pitcher throws a baseball without spin with a velocity of 20 m/s. If the ball has a circumference of 225 mm, calculate the drag force on the ball in air at 30C.
-
Calculate the moment at the base of a flagpole caused by a wind of 150 km/h. The pole is made of three sections, each 3 m long, of different-size Schedule 80 steel pipe. The bottom section is DN 150,...
-
Determine the terminal velocity (see Section 2.6.4, Chapter 2 ) of a 75-mm-diameter sphere made of solid aluminum (specific weight = 26.6 kN/m 3 ) in free fall in (a) castor oil at 25C, (b) water at...
-
5) Given the below stress field determine the body force distribution required for equilibrium. Oxx = Oyy = x + y xy = 5z+2y xz = x2 1 = xz + xy 2x3+ y yz = 0 zz = 4y - 23
-
On the Venn diagram, shade the region A' n B U B [1pt.] (b) The events A and B are such that P(An 8')=0.2 and P(AUB) 0.9. Find P(A|B). [4 pts.] 3. (a) The events A and B are such that P(A) = 0.65,...
-
r(t) is the position vector of a moving particle. Graph the curve and the velocity and acceleration vectors at the indicated time. Find the speed at that time. 1 r(t) = 1 + j; 1= 1 +

Study smarter with the SolutionInn App


