The vapor pressure of dichloromethane is measured as a function of temperature, and the results are tabulated.
Question:
The vapor pressure of dichloromethane is measured as a function of temperature, and the results are tabulated. From the results, determine the heat of vaporization of dichloromethane.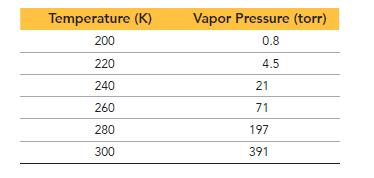
Transcribed Image Text:
Temperature (K) 200 220 240 260 280 300 Vapor Pressure (torr) 0.8 4.5 21 71 197 391
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 66% (3 reviews)
To find the heat of vaporization use an Excel sprea...View the full answer

Answered By

Ashington Waweru
I am a lecturer, research writer and also a qualified financial analyst and accountant. I am qualified and articulate in many disciplines including English, Accounting, Finance, Quantitative spreadsheet analysis, Economics, and Statistics. I am an expert with sixteen years of experience in online industry-related work. I have a master's in business administration and a bachelor’s degree in education, accounting, and economics options.
I am a writer and proofreading expert with sixteen years of experience in online writing, proofreading, and text editing. I have vast knowledge and experience in writing techniques and styles such as APA, ASA, MLA, Chicago, Turabian, IEEE, and many others.
I am also an online blogger and research writer with sixteen years of writing and proofreading articles and reports. I have written many scripts and articles for blogs, and I also specialize in search engine
I have sixteen years of experience in Excel data entry, Excel data analysis, R-studio quantitative analysis, SPSS quantitative analysis, research writing, and proofreading articles and reports. I will deliver the highest quality online and offline Excel, R, SPSS, and other spreadsheet solutions within your operational deadlines. I have also compiled many original Excel quantitative and text spreadsheets which solve client’s problems in my research writing career.
I have extensive enterprise resource planning accounting, financial modeling, financial reporting, and company analysis: customer relationship management, enterprise resource planning, financial accounting projects, and corporate finance.
I am articulate in psychology, engineering, nursing, counseling, project management, accounting, finance, quantitative spreadsheet analysis, statistical and economic analysis, among many other industry fields and academic disciplines. I work to solve problems and provide accurate and credible solutions and research reports in all industries in the global economy.
I have taught and conducted masters and Ph.D. thesis research for specialists in Quantitative finance, Financial Accounting, Actuarial science, Macroeconomics, Microeconomics, Risk Management, Managerial Economics, Engineering Economics, Financial economics, Taxation and many other disciplines including water engineering, psychology, e-commerce, mechanical engineering, leadership and many others.
I have developed many courses on online websites like Teachable and Thinkific. I also developed an accounting reporting automation software project for Utafiti sacco located at ILRI Uthiru Kenya when I was working there in year 2001.
I am a mature, self-motivated worker who delivers high-quality, on-time reports which solve client’s problems accurately.
I have written many academic and professional industry research papers and tutored many clients from college to university undergraduate, master's and Ph.D. students, and corporate professionals. I anticipate your hiring me.
I know I will deliver the highest quality work you will find anywhere to award me your project work. Please note that I am looking for a long-term work relationship with you. I look forward to you delivering the best service to you.
3.00+
2+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
a. The following data have been reported for the vapor pressure of ethanol as a function of temperature. Use these data to calculate the heat of vaporization of ethanol at 17.33C. b. Ackermann and...
-
The Dew Point The vapor pressure of water (see Problem 18.88) decreases as the temperature decreases. If the amount of water vapor in the air is kept constant as the air is cooled, a temperature is...
-
We have learned that the enthalpy of vaporization of a liquid is generally a function of temperature. If we wish to take this temperature variation into account, we cannot use the ClausiusClapeyron...
-
Terminology Key: Key Word List Describe Explain What to do In bulleted, numbered or paragraph form, provide a number of consecutive items-if paragraph form, use commas (,) to separate items In...
-
Why is interest paid on amounts borrowed from banks and other lenders considered to be an operating activity while the amounts borrowed are financing activities?
-
The voltage response of a network to a unit step input is the response critically damped? V,(s) s(s? + 8s + 18) 10
-
How long have you been working in your current position?
-
The adjusted trial balance columns of the worksheet for Alshwer Company, owned by M. Alshwer, are as follows. Instructions (a) Complete the worksheet by extending the balances to the financial...
-
Float is defined as the difference between the balance shown on the books and the balance in the bank account. A lag often occurs between the time receipts and disbursements are recorded on the...
-
An electrostatic potential map for acetonitrile (CH 3 CN), which is polar, is shown here: From this map, determine the geometry for how two acetonitrile molecules would interact with each other....
-
What are the main properties of liquids (in contrast to gases and solids)?
-
West Coast Real Estate Management is expanding operations, and the firms president, Mike Mayberry, is trying to make a decision about new office space. The following are the firms options: Maple...
-
Discuss how the characteristics of the nursing profession assist with the process of delegation and the need for delegation and what are 2 barriers to delegation.
-
Does product quality influence what you purchase? Do you believe that good quality equals customer satisfaction?
-
How did the cognitive dissonance make you feel? Why? How did you resolve the dissonance? Did you change your attitude, change your behavior, add new cognitions, or something else? Did you use...
-
After reading the Texas Board of Nursing "Delegation Packet, describe a situation that can occur in a work setting, or that could potentially occur, that demonstrates the importance of following the...
-
Do you believe compensation should be confidential? Should all position compensations be confidential, or only select positions (for example, should the average employees compensation be...
-
Carlsbad Enterprises has a capacity to produce 400,000 computer cases per year. The company is currently producing and selling 320,000 cases per year at a selling price of $80 per case. The cost of...
-
Factor and simplify, if possible. Check your result using a graphing calculator. 3 cot 2 + 6 cot + 3
-
When the temperature is at 30°C, the A-36 steel pipe fits snugly between the two fuel tanks. When fuel flows through the pipe, the temperatures at ends A and B rise to 130°C and 80°C,...
-
The bar has a cross-sectional area A, length L, modulus of elasticity E, and coefficient of thermal expansion a. The temperature of the bar changes uniformly along its length from T A at A to T B at...
-
The device is used to measure a change in temperature. Bars AB and CD are made of A-36 steel and 2014-T6 aluminum alloy, respectively. When the temperature is at 75°F, ACE is in the horizontal...
-
When computing the amount of interest cost to be capitalized, the concept of avoidable interest' refers to a. the total interest cost actually incurred b. a cost of capital charge for stockholders...
-
Question The digital microwave radio system shown in Figure 2 operates in... The digital microwave radio system shown in Figure 2 operates in the 18GHz radio frequency band and provides 2x2 Mbps...
-
Tenneson Corporation's cost of goods manufactured for the just completed month was $151,000 and its inventories were as follows: Beginning Ending Work in process inventory $ 63,000 $ 66,000 Finished...

Study smarter with the SolutionInn App


