The VSEPR model is useful in predicting bond angles for many compounds. However, as we have seen,
Question:
The VSEPR model is useful in predicting bond angles for many compounds. However, as we have seen, other factors (such as type of bond and atomic radii) may also influence bond angles.
Consider that data for bond angles in related species in the tables and answer the questions.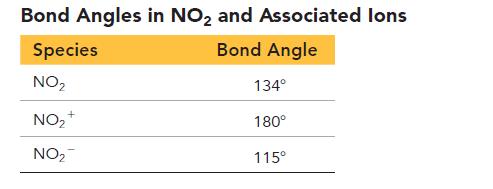
a. Draw Lewis structures for all of the species in the Bond Angles in NO2 and Associated Ions Table.
b. Use the Lewis structures from part a to explain the observed bond angles in NO2 and its associated ions.
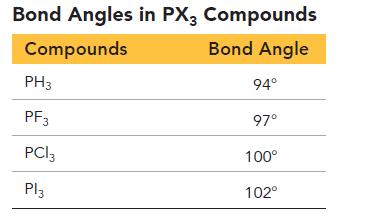
c. Draw Lewis structures for all of the species in the Bond Angles in PX3 Compounds Table.
d. Make your own table showing the atomic radii of H, F, Cl, and I.
e. Use your answers to parts c and d to explain the observed bond angles in PH3, PF3, PCl3, and PI3.
Step by Step Answer:






