Use formal charges to identify the better Lewis structure. H H-S-C-H 1 H H T H-C-S-H I
Question:
Use formal charges to identify the better Lewis structure.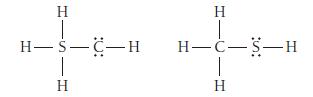
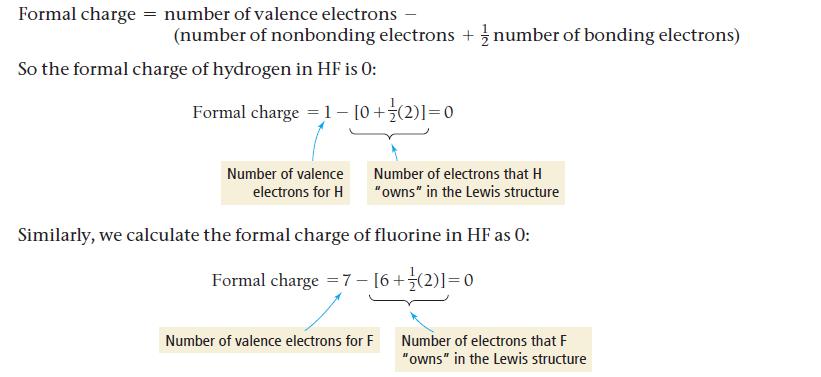
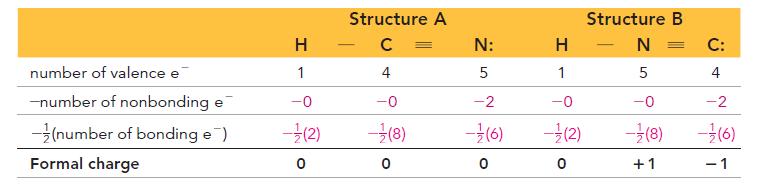
Transcribed Image Text:
H H-S-C-H 1 H H T H-C-S-H I H
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (QA)
To determine the better Lewis structure based on formal charges we look for the structure in which t...View the full answer

Answered By

Grace Igiamoh-Livingwater
I am a qualified statistics lecturer and researcher with an excellent interpersonal writing and communication skills. I have seven years tutoring and lecturing experience in statistics. I am an expert in the use of computer software tools and statistical packages like Microsoft Office Word, Advanced Excel, SQL, Power Point, SPSS, STATA and Epi-Info.
5.00+
1+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
A long conducting wire made of anti-matter carries a uniform current per unit area. The anti-electrons (each carries charge +1.602 x 10-19 C ) with speed 4.862 m/s (the speed and magnitude of the...
-
In N 2 O, nitrogen is the central atom, and the oxygen atom is terminal. In OF 2 , however, oxygen is the central atom. Use formal charges to explain why. Formal charge = number of valence electrons...
-
Use formal charge to identify the better Lewis structure. H H-C= H H=C
-
Provide the appropriate statute for your answer (format style - IRAC - Issue, Rule, Application and Conclusion.) what are your thought? Problem Scenario- Earnest is married to Janice. Earnest and...
-
What is SQL? How is SQL like an Access query? How is it different?
-
Below are transactions that took place in Placid Company during the past year: a. Equipment was purchased. b. A cash dividend was declared and paid. c. Accounts receivable decreased. d. Short-term...
-
CGI Federal, Inc., is a corporation that provides a number of services to the United States Passport Agency, included the processing of passport application. Passport applicants must submit sensitive...
-
On October 1, Little Bobby Corporations stockholders equity is as follows. Common stock, $5 par value ............ $400,000 Paid-in capital in excess of parcommon stock ... 25,000 Retained earnings...
-
Ho: 5. Now let's consider the effects of comparing responses across samples. Suppose that groups 1 and 2 used the same people, and that responses for individuals 1 to 8 are listed in each row....
-
How important is the resonance structure shown here to the overall structure of carbon dioxide? Explain. :0=C:
-
Write a Lewis structure that obeys the octet rule for each ion. Include resonance structures if necessary and assign formal charges to each atom. a. CIO3 b. ClO4 c. NO3 + d. NH4
-
James Moore was interested in purchasing a used Mack truck from Worldwide, but he was concerned about the eighteen-speed transmission. Worldwides sales representative assured Moore that the...
-
Under very particular conditions, banks would like to borrow from the Fed and, rather than use these borrowed funds to make loans, keep them in the form of excess reserves. What would be the effect...
-
As the CEO of a leading bank in the City of London, you have decided to directly meet protestors in the Occupy London movement. What will you say?
-
Suppose Japan has a GDP of $5 trillion, and that its national savings rate is 25%. a) Calculate Japans national saving. b) Calculate Japans government saving if private saving is $800 billion.
-
Suppose you take out a loan at your local bank and the nominal interest rate is 12%. The bank expects the inflation rate to be 4% during the life of your loan. a) What is the banks ex ante real...
-
Uruguay has implemented the One Laptop per Child (OLPC) initiative in which one laptop is given to every child and teacher in a public primary school. Comment on the effects of this program on the...
-
What factors determine a countrys terms of trade?
-
For liquid water the isothermal compressibility is given by; where r and b are functions of temperature only. If 1 kg of water is compressed isothermally and reversibly from I to 500 bar at 60(C. how...
-
Denver, Colorado, is called the Mile-High City because it is located at an elevation of approximately 5200 ft. Assuming that the sea-level pressure is 101.3 kPa(abs), what would be the approximate...
-
The barometric pressure is reported to be 28.6 in of mercury. Calculate the atmospheric pressure in psia.
-
A barometer indicates the atmospheric pressure to be 30.65 in of mercury. Calculate the atmospheric pressure in psia.
-
A queue system has 8 customers on the queue and the waiting time in the queue is 1.3 hour. If the expected system utilization factor is 90% what should the mean service rate of the system per hour?
-
What needs does your product net hubb satisfy and what kind of consumer products do it sell?
-
Analyse the KFC fast food brand "s company description, Target segment, competitive advantage, marketing plan objectives, Mission and goals( non financial goals and financial goals), as well as...

Study smarter with the SolutionInn App


