Use standard enthalpies of formation to calculate H rxn for each reaction. a. CH4(g) + H(g) CH6(8)
Question:
Use standard enthalpies of formation to calculate ΔH°rxn for each reaction.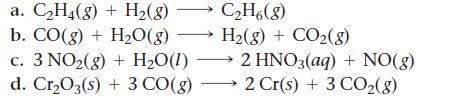
Transcribed Image Text:
a. C₂H4(g) + H₂(g) C₂H6(8) b. CO(g) + H₂O(g) →→→ H₂(g) + CO₂(g) c. 3 NO₂(g) + H₂O(1) d. Cr₂O3(s) + 3 CO(g) 2 HNO3(aq) + NO(g) 2 Cr(s) + 3 CO₂(8)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (1 review)
a 1371 ...View the full answer

Answered By

Daniel Kimutai
I am a competent academic expert who delivers excellent writing content from various subjects that pertain to academics. It includes Electronics engineering, History, Economics, Government, Management, IT, Religion, English, Psychology, Sociology, among others. By using Grammarly and Turnitin tools, I make sure that the writing content is original and delivered in time. For seven years, I have worked as a freelance writer, and many scholars have achieved their career dreams through my assistance.
5.00+
1+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Calculation of H rxn for Part I 0 1. Assume the heat capacity of the final solution is 4.184 J K-1 g-1. Using the final mass of the solution in the calorimeter, calculate q contents from equation...
-
Suppose that you sell a product that is demanded by two different segments of the population (call them X and Y). Suppose that the inverse demand from segment X is Px = 200-2Qx and the inverse demand...
-
An important step in the production of sulfuric acid is the oxidation of SO 2 to SO 3 . Formation of SO 3 from the air pollutant SO 2 is also a key step in the formation of acid rain. (a) Use...
-
The bookkeeper of Cinnamon Ltd. who is usually responsible for the bank reconciliation is on holiday. Before she left she asked you to do this task for her. On March 31, you are given the bank...
-
The cash records of Haig Company show the following. For July: 1. The June 30 bank reconciliation indicated that deposits in transit total $750. During July the general ledger account Cash shows...
-
A hydrogen atom in an n = 2, l = 1, m1 = -1 state emits a photon when it decays to an n = 1, l = 0, m1 = 0 ground state. (a) In the absence of an external magnetic field, what is the wavelength of...
-
Calculate the energy density versus temperature very early in the universe when the temperatures were above \(k T=300 \mathrm{MeV}\). At those temperatures, quarks and gluons were released from...
-
Consider the balance sheets and selected data from the income statement of Keith Corporation that appear below and on shown below. Keith Corporation Income Statement Data (2012) Depreciation expense...
-
What are the reasons to use a relational database? What data does not fit well in the relational model?
-
Write an equation for the formation of each compound from its elements in their standard states, and find H rxn for each. a. NO 2 (g) b. MgCO 3 (s) c. C 2 H 4 (g) d. CH 3 OH(l)
-
Write an equation for the formation of each compound from its elements in their standard states, and find H f for each in Appendix IIB. a. NH 3 (g) b. CO 2 (g) c. Fe 2 O 3 (s) d. CH 4 (g)
-
At what temperature does a Celsius thermometer give the same numerical reading as a Fahrenheit thermometer?
-
Discuss specific strategies and insights from CAS and other organizational theories that can help improve the adaptability and resilience of the healthcare team in a dynamic environment.
-
Explain who was Charlemagne? Discuss several of his accomplishments. In a separate paragraph, separated by a blank line...Discuss what you think was his most important accomplishment and why you...
-
There are various known branches of psychology. List these major branches of psychology.?
-
Answer to the following: Discuss how a company's internal environment might affect the development of the corporate strategy. Include product life cycle, personnel, and organizational structure in...
-
1)What makes psychology a science? 2) Do you agree with Stanovich that psychology is "alive and well" among the sciences? Why or why not? Stanovich: how to think straight about psychology
-
The internal control procedures in Valentine Company provide that: 1. Employees who have physical custody of assets do not have access to the accounting records. 2. Each month, the assets on hand are...
-
What is the mode?
-
Explain why T 1 T 2 .
-
Explain why two magnetic fields, a static field and a radiofrequency field, are needed to carry out NMR experiments. Why must the two field directions be perpendicular?
-
Explain the difference in the mechanism that gives rise to through-space dipoledipole coupling and through-bond coupling.
-
Jake is an IRS tax law - certified volunteer preparer at a VITA / TCE site. When preparing a return for Jill, Jake learns that Jill does not have a bank account to receive a direct deposit of her...
-
1. Calculate the following: a. What was the percentage change in total operating revenues from FY2015 to FY2019? b. What was the percentage change in total expenses (operating + non-operating) from...
-
Why does a more accurate cost for a product matter? Shouldn't the price be set based on market and competitive factors? What causes a product or customer to be highly unprofitable? When would a...

Study smarter with the SolutionInn App


