Without doing any calculations, determine the sign of S sys for each chemical reaction. a. Mg(s) +
Question:
Without doing any calculations, determine the sign of ΔSsys for each chemical reaction.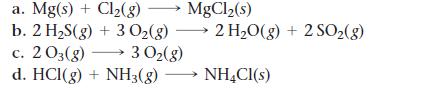
Transcribed Image Text:
a. Mg(s) + Cl₂(g) →→→ MgCl₂(s) b. 2 H₂S(g) + 3 O₂(g) → 2 H₂O(g) + 2 SO₂(g) c. 203(g) d. HCI(g) + NH3(g) NH4Cl(s) 3 O₂(g)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 66% (3 reviews)
To determine the sign of Ssys the change in entropy of the system for each chemical reaction without ...View the full answer

Answered By

ANDREW KIPRUTO
Academic Writing Expert
I have over 7 years of research and application experience. I am trained and licensed to provide expertise in IT information, computer sciences related topics and other units like chemistry, Business, law, biology, biochemistry, and genetics. I'm a network and IT admin with +8 years of experience in all kind of environments.
I can help you in the following areas:
Networking
- Ethernet, Wireless Airmax and 802.11, fiber networks on GPON/GEPON and WDM
- Protocols and IP Services: VLANs, LACP, ACLs, VPNs, OSPF, BGP, RADIUS, PPPoE, DNS, Proxies, SNMP
- Vendors: MikroTik, Ubiquiti, Cisco, Juniper, HP, Dell, DrayTek, SMC, Zyxel, Furukawa Electric, and many more
- Monitoring Systems: PRTG, Zabbix, Whatsup Gold, TheDude, RRDtoo
Always available for new projects! Contact me for any inquiries
4.30+
1+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Without doing any calculations, determine the sign of S sys for each chemical reaction. a. 2 KClO 3 (s) 2 KCl(s) + 3 O 2 ( g) b. CH 2 = CH 2 ( g) + H 2 ( g) CH 3 CH 3 (g) c. Na(s) + 1/2 Cl 2 (g) ...
-
Planning is one of the most important management functions in any business. A front office managers first step in planning should involve determine the departments goals. Planning also includes...
-
Managing Scope Changes Case Study Scope changes on a project can occur regardless of how well the project is planned or executed. Scope changes can be the result of something that was omitted during...
-
Which is false? The Chinese government is reluctant to let the yuan appreciate against the US dollar because: Appreciation of the yuan would increase the price of real estate for young Chinese...
-
Discuss when qualitative research may be more effective than quantitative research.
-
What is the logic in charging administrative costs based on total time to project completion?
-
In 1951, DuPont began using the chemical perfluorooctanoic acid to manufacture Teflon. Due to the dangerous nature of the chemical, DuPont was given special instructions by its supplier to dispose of...
-
Latona Hardware Store completed the following merchandising transactions in the month of May. At the beginning of May, the ledger of Latona showed Cash of $5,000 and Owners Capital of $5,000. May 1...
-
A combined solar and auxiliary energy system is used to meet the same load as in Example 12.5. The total cost of the system to cover 65% of the load (solar fraction) is $20,000. The owner will pay a...
-
Without doing any calculations, determine the signs of S sys and S sur r for each chemical reaction. In addition, predict under what temperatures (all temperatures, low temperatures, or high...
-
Calculate the change in entropy that occurs in the system when 55.0 g of water vaporizes from a liquid to a gas at its boiling point (100.0 C). See Table 12.7 for heats of vaporization. TABLE 12.7...
-
In Exercises 1524, use Greens Theorem to evaluate the line integral. [ (x - y) dx + 2xy dy C: r = 1 + cos 0
-
Discuss the topic - Local, regional and global development issues. 2. Discuss some of the development issues faced on the regional and global scene. 3.. Name and describe the functions of some of the...
-
Explain why? globalization on communication could possible lead to mash or mash up of civilization where in certain customs of a person or place would combine and amalgamate with one another...
-
Identify your choice of the top five elements of a human resources strategy that link to an organization's overall strategic goals. Support your selections using this week's resources. Also, support...
-
Prepare to communicate complex information and negotiate outcomes. What information (related to your selected workplace project or issue) must be conveyed and negotiated? With whom will the...
-
complex and sophisticated technologies have created problems for society because they have outstripped the human ability to control them; the naked ape is not able to act responsibly with the...
-
How the changes in the lending regulatory environment, particularly with the passage of the Dodd-Frank Act, has impacted the banks ability to lend money to businesses for capital projects and...
-
The packaging division of a company having considered several alternative package designs for the company's new product has finally brought down their choices to two designs of which only one has to...
-
Determine the Fourier transform of the function Make a sketch of F{E(x)}. Discuss its relationship to Fig. 11.11. Fig. 11.11 |x| < L |x|> L SEo sinkp.x E(x) = (a) f(x) (a) F(k) = A(k) %3D 5 -d/2 0...
-
Determine the Fourier transform of Make a sketch of it. |x| < L |x| > L S sin-kpx f(x) =
-
Determine the Fourier transform of Make a sketch of F(Ï), then sketch its limiting form as T ±. Scos w,t |1| T
-
Martinez Ltd. issued a $1,060,000, 10-year bond at par on January 1, 2023. The bond paid 10% interest each January 1 and July 1. The company's year end was September 30. Prepare the journal entries...
-
Sophia is the director for her company's South Korean operations. She is interviewing candidates to fill the position of factory manager at the location in Seoul. Sophia carefully considers the...
-
How do you ensure the robustness and reliability of data mining models in dynamic and evolving environments, employing techniques such as online learning, model adaptation, and concept drift...

Study smarter with the SolutionInn App


