Question: Calculate the change in entropy that occurs in the system when 55.0 g of water vaporizes from a liquid to a gas at its boiling
Calculate the change in entropy that occurs in the system when 55.0 g of water vaporizes from a liquid to a gas at its boiling point (100.0 °C). See Table 12.7 for heats of vaporization.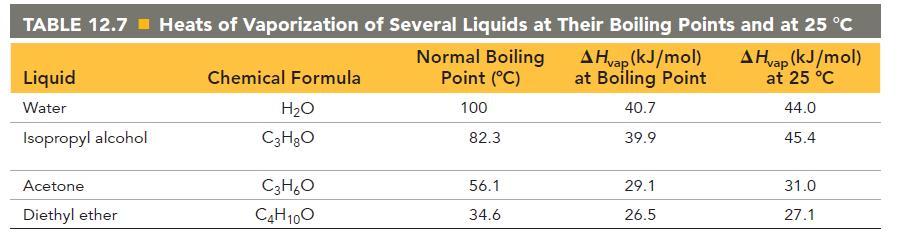
TABLE 12.7 Heats of Vaporization of Several Liquids at Their Boiling Points and at 25 C A Hvap (kJ/mol) at Boiling Point A Hvap (kJ/mol) at 25 C 44.0 45.4 Liquid Water Isopropyl alcohol Acetone. Diethyl ether Chemical Formula HO C3HO C3HO C4H10O Normal Boiling Point (C) 100 82.3 56.1 34.6 40.7 39.9 29.1 26.5 31.0 27.1
Step by Step Solution
3.38 Rating (160 Votes )
There are 3 Steps involved in it
To calculate the change ... View full answer

Get step-by-step solutions from verified subject matter experts


