Without doing any calculations, determine the signs of S sys and S sur r for each chemical
Question:
Without doing any calculations, determine the signs of ΔSsys and ΔSsurr for each chemical reaction. In addition, predict under what temperatures (all temperatures, low temperatures, or high temperatures), if any, the reaction is spontaneous.
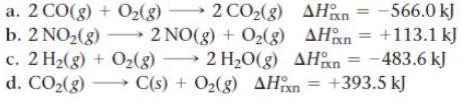
Transcribed Image Text:
a. 2 CO(g) + O(g) 2 CO(g) b. 2 NO(g) 2NO(g) + O(g) AH AH = -566.0 kJ = +113.1 kJ c. 2 H(g) + O(g) 2HO(g) AHxn=-483.6 kJ d. CO(g) C(s) + O(g) AHxn = +393.5 kJ
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (1 review)
Without doing any calculations we can determine the signs of Ssys and Ssurr for each chemical reacti...View the full answer

Answered By

Evans Cherono
I am an Information Technology Graduate and willing to work on any computer science or IT work to ensure I do my best all the time.
5.00+
2+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Without doing any calculations, determine the signs of S sys and S sur r for each chemical reaction. In addition, predict under what temperatures (all temperatures, low temperatures, or high...
-
Read the case study "Southwest Airlines," found in Part 2 of your textbook. Review the "Guide to Case Analysis" found on pp. CA1 - CA11 of your textbook. (This guide follows the last case in the...
-
Managing Scope Changes Case Study Scope changes on a project can occur regardless of how well the project is planned or executed. Scope changes can be the result of something that was omitted during...
-
3. Questions A venture capitalist (VC) is willing to invest 100m for 20% ownership of a start-up that is looking to achieve scale. All existing shares are common shares, and this deal would result in...
-
Sampling presents some major problems in market research. Discuss.
-
Consider one or more projects (from this course or elsewhere) that you understand reasonably well. Identify situations where information learned from a later task of the project becomes important to...
-
James Lewis, a resident of Kentucky, sustained an injury while operating a Caterpillar bulldozer. He filed suit against Caterpillar, a company incorporated in Delaware but with its principal place of...
-
For years The Glass Slipper restaurant has operated in a resort community near a popular ski area of New Mexico. The restaurant is busiest during the first 3 months of the year, when the ski slopes...
-
Wood Jojo is a carpenter and now he needs X metres of wood. He is now in a forest with N trees with integer height in metres, A 1 , A 2 , . . . , A N . He has a special cutting device. The device can...
-
Without doing any calculations, determine the sign of S sys for each chemical reaction. a. 2 KClO 3 (s) 2 KCl(s) + 3 O 2 ( g) b. CH 2 = CH 2 ( g) + H 2 ( g) CH 3 CH 3 (g) c. Na(s) + 1/2 Cl 2 (g) ...
-
Without doing any calculations, determine the sign of S sys for each chemical reaction. a. Mg(s) + Cl(g) MgCl(s) b. 2 HS(g) + 3 O(g) 2 HO(g) + 2 SO(g) c. 203(g) d. HCI(g) + NH3(g) NH4Cl(s) 3 O(g)
-
In Exercises 3150, find f + g, f - g, fg, and f/g. Determine the domain for each function. f(x) = x, g(x) = x - 4
-
Assume that you are a recently hired department manager within a sizeable healthcare provider organization. You came from a small organization where you and other managers enjoyed a cooperative...
-
How is the representation of a character effected by the genre? For example, in chick flicks, the female protagonist is often portrayed to be bubbly and hyper-feminine. Further, how does the...
-
Identify products with shorter life cycle and discuss. Why is the median a better representative of the dataset than the mean? What is impulse purchase? How does this contribute to retail marketing?
-
Describe how the Communication and Negotiation course will help you in the future and if you find this course to be useful? 2. Please describe how your experience was in the Negotiation Game that was...
-
Assess the likely impact of the changes resulting from the internationalizing of the enterprise on the organization's human resources.
-
Go to the Bureau of Labor Statistics website, www.bls.gov/news.release/empsit.toc.htm, and click on "Employment Situation Summary" to get the most up-to-date summary of unemployment in the U.S. or...
-
Solve the relation Exz:Solve therelation ne %3D
-
A long narrow slit 0.10 mm wide is illuminated by light of wavelength 500 nm coming from a point source 0.90 m away. Determine the irradiance at a point 2.0 m beyond the screen when the slit is...
-
A long horizontal narrow slit of width 0.70 mm is illuminated with 600-nm light. A point-P, 1.0 m away from the aperture screen, is opposite the lower edge of the screen. If 100 W/m 2 arrives at P...
-
A long narrow horizontal opaque rectangular object of width 0.70 mm is illuminated by 600-nm light. Consider a point-P, at the level of the lower edge of the object, 1.0 m from it. Determine the...
-
HUDSON COMPANY Contribution Margin Income Statement For Year Ended December 31 Sales (10,400 units at $280 each) Variable costs (10,400 units at $210 each) Contribution margin Fixed costs Income $...
-
What type of HR supply model utilizes a series of matrices that detail the various patterns of movement to and from the various jobs in an organization?
-
When do you have evidence that your message was successful? At what part of the message?

Study smarter with the SolutionInn App


