At 40C, H 2 O 2 (aq) will decompose according to the following reaction: The following data
Question:
At 40°C, H2O2(aq) will decompose according to the following reaction:
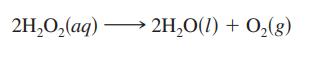
The following data were collected for the concentration of H2O2 at various times.
![Time (s) 0 2.16 X 104 4.32 X 104 [HO] (mol/L) 1.000 0.500 0.250](https://dsd5zvtm8ll6.cloudfront.net/images/question_images/1699/7/0/7/417654f7a199abbf1699707418487.jpg)
a. Calculate the average rate of decomposition of H2O2 between 0 and 2.16 × 104 s. Use this rate to calculate the average rate of production of O2(g) over the same time period.
b. What are these rates for the time period 2.16 × 104 s to 4.32 × 104 s?
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Chemistry An Atoms First Approach
ISBN: 9781305079243
2nd Edition
Authors: Steven S. Zumdahl, Susan A. Zumdahl
Question Posted:





