Order the following solids (ad) from least soluble to most soluble. Ignore any potential reactions of the
Question:
Order the following solids (a–d) from least soluble to most soluble. Ignore any potential reactions of the ions with water.
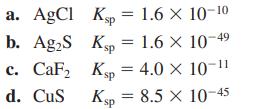
Transcribed Image Text:
a. AgCl Kp = 1.6 × 10-10 Ksp = 1.6 × 10- b. Ag₂S c. CaF₂ d. CuS Ksp = 4.0 × 10-¹¹ Ksp = 8.5 X 10-45
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (2 reviews)
Note When a salt has a larger Ksp value its solubility will be hi...View the full answer

Answered By

David Muchemi
I am a professional academic writer with considerable experience in writing business and economic related papers. I have been writing for my clients who reach out to me personally after being recommended to me by satisfied clients.
I have the English language prowess, no grammatical and spelling errors can be found in my work. I double-check for such mistakes before submitting my papers.
I deliver finished work within the stipulated time and without fail. I am a good researcher on any topic especially those perceived to be tough.
I am ready to work on your papers and ensure you receive the highest quality you are looking for. Please hire me to offer my readily available quality service.
Best regards,
4.60+
27+ Reviews
61+ Question Solved
Related Book For 

Chemistry An Atoms First Approach
ISBN: 9781305079243
2nd Edition
Authors: Steven S. Zumdahl, Susan A. Zumdahl
Question Posted:
Students also viewed these Sciences questions
-
Assuming that the solubility of Ca 3 (PO 4 ) 2 (s) is 1.6 10 -7 mol/L at 25 C, calculate the K sp for this salt. Ignore any potential reactions of the ions with water.
-
When a pure substance is placed in contact with water, there are three possible outcomes. The substance may do nothing that is, the substance does not dissolve and no visible change takes place. The...
-
Part 1 a. Ammonia, NH 3 , is a weak electrolyte. It forms ions in solution by reacting with water molecules to form the ammonium ion and hydroxide ion. Write the balanced chemical reaction for this...
-
Perform the indicated operations and simplify the result. Leave your answer in factored form. X x - 3 x + 1 x2 + 5x 24
-
What type of bailment relationship was created when Denai agreed to store Finneys boat? What degree of care was Denai required to exercise in storing the boat? Vanessa Denai purchased forty acres of...
-
Rayleigh's dissipation function is used to generate a a. stiffness matrix b. damping matrix c. mass matrix
-
The car bumper is made of polycarbonate polybutylene terephthalate. If \(E=2.0 \mathrm{GPa}\), determine the maximum deflection and maximum stress in the bumper if it strikes the rigid post when the...
-
Stephen Hall is developing a program in supply chain management certification for managers. Hall has listed a number of activities that must be completed before a training program of this nature...
-
Do a program that translates a word from English to Pig Latin. Get the word from the user. Pig Latin is a nonsense language. To make a word in Pig Latin, you remove the first letter of the English...
-
You, CPA, work as an associate with Campbell and Associates LLP, a financial and business advisory firm. The board of directors of Pembroke Pulp and Paper Inc. (PPPI) has engaged your firm once again...
-
A 50.0-mL sample of 0.00200 M AgNO 3 is added to 50.0 mL of 0.0100 M NaIO 3 . What is the equilibrium concentration of Ag + in solution? (K sp for AgIO 3 is 3.0 10 -8 .)
-
Calculate the solubility of Co(OH) 2 (s) (K sp = 2.5 10 -16 ) in a buffered solution with a pH of 11.00.
-
Describe the transition that employees go through when they are promoted to management.
-
Determine the maximum and minimum resultant of two forces having magnitude of 10 N and 8N respectively.
-
Identify the two modes of tax classifications and their respective tax systems in most open economies? Discuss the economic implications of the above (5a) tax systems in developing economies
-
Kirinyaga Hydraulic Contractors in partnership with the World Bank are embarking on a water project in Mukurweini Division. They are contemplating to gauge the benefits of the project through cost-...
-
Explain demographic transition theory as was proposed by Warren Thompson and highlight the stages of development transition
-
Using simplex method, solve the following Linear Programming problem and interpret the solutions Maximize II 2x, +12x2 +8x3 Subject to the constraints 2x+2x+x, 100, x-2x+5x, 80 10x, +5x+4x, 300, xxx...
-
It is generally assumed that alcohol consumption tends to make drinkers more impulsive. However, a recent study in the journal Alcohol and Alcoholism may contradict this assumption. The study took a...
-
When a company has a contract involving multiple performance obligations, how must the company recognize revenue?
-
Identify the reagents that you would use to accomplish each of the following transformations: (a) (b) H.
-
Predict the major product for each of the following transformations: (a)
-
Identify the reagents that you would use to accomplish each of the following transformations (you will also need to use reactions from previous chapters). (a) (b) (c) Br Br OH
-
A teacher has surveyed 100 students on which superpower they would most like to have. The following table shows the collected data for those students: Superpower Male Female TOTAL Fly 26 12 38...
-
a) Compute the time complexity of the following code with detailed analysis (5 marks) int a[10]={2,4,6,8,10,12,14,16,18,20}; for(int i=0; i
-
1 ) By the Du Pont formula, we know ROA can be decomposed into two financial ratios. What are these ratios? 2 ) Based on two financial ratios, we can identify two important relationships. 3 ) By...

Study smarter with the SolutionInn App


