Without using Fig. 3.4, predict which bond in each of the following groups will be the most
Question:
Without using Fig. 3.4, predict which bond in each of the following groups will be the most polar.
Fig. 3.4
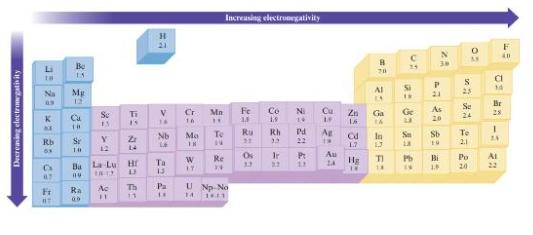
a. C-H, Si-H, Sn-H
b. Al-Br, Ga-Br, In-Br, Tl-Br
c. C-O or Si-O
d. O-F or O-CI
Transcribed Image Text:
Decreasing electronegativity Li Na * KE Rb 63 C 67 Fi #T Be 15 7 * =2 E Mg Se Y 12 Se 13 Ra 00 Ti 15 Ba La Lu Hr Bu Ac 11 Z 14 O D Th 11 H 21 V 14 Nb 23 22 4: Ta Pa Cr 16 9: Mo 18 W 17 Mn H Increasing electronegativity To 14 Re 14 U Np No 14 16.11 Fe 2= 2 82 Ru 3.3 Co 19 Rh 33 N Cu 2: 23 42 Pd 22 8: 2:33 1.3 Ag 1r P: Au 3.2 39 89 22 C 25 20 Al 18 D Ga Ge 23 In Sa 13 Zn 16. 1.7 Hg ROBS an Rd TI 14 IN a £: Pb N 30 P 21 As 20 Sb 19 Bi 1.8 0 S 23 To 2.1 AV Se 24 Po 20 a 14 Br 28 -: F 40 24 Ai
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (3 reviews)
a CH SiH SnH Carbon is more electronegative than silicon and ...View the full answer

Answered By

Isaiah Mutinda
As a graduate with Bs in Maths and Computer Science and having worked as a freelance full stack software developer for 3 years running I believe I have what it takes to conformable tutor and mentor a student to a professional developer also.
5.00+
2+ Reviews
10+ Question Solved
Related Book For 

Chemistry An Atoms First Approach
ISBN: 9781305079243
2nd Edition
Authors: Steven S. Zumdahl, Susan A. Zumdahl
Question Posted:
Students also viewed these Sciences questions
-
Use the electro negatively table (Figure) to predict which bond in each of the following sets is more polar, and indicate the direction of bond polarity for each compound. (a) H3C ? C1 OR C1 ? C1 (b)...
-
Without using Fig. 13.3, predict which bond in each of the following groups is the most polar. a. COF, SiOF, GeOF b. POCl, SOCl c. SOF, SOCl, SOBr d. TiOCl, SiOCl, GeOCl e. COH, SiOH, SnOH f. AlOBr,...
-
Without using Fig. 3.4, predict which bond in each of the fol- lowing groups will be the most polar. Data in Fig. 3.4 a. C-F, Si-F, Ge-F b. P-Cl or S-Cl c. S-F, S-Cl, S-Br d. Ti-Cl, Si-Cl, Ge-Cl...
-
Do you have convincing evidence of sufficient computer skills to engage in online discussion forums, access online library resources, engage in online videoconferencing, and utilize word processing,...
-
You have been asked to evaluate possible sites for an Asian production facility that will manufacture your firms products and sell them to the Asian market. What real exchange rate considerations...
-
Why are Interpretations of the AICPA Code written?
-
Which type of audit involves a review of general and application controls, with a focus on determining if there is compliance with policies and adequate safe guarding of assets? a. information...
-
Any given cost-information system is a function of a number of design choices. For product-costing purposes, one such design choice relates to how overhead, particularly fixed manufacturing overhead,...
-
A campus deli serves 350 customers over its busy lunch period from 11:30 a.m. to 1:30 p.m. A quick count of the number of customers waiting in line and being served by the sandwich makers shows that...
-
Which of the following incorrectly shows the bond polarity? Show the correct bond polarity for those that are incorrect.
-
Describe the type of bonding that exists in the Cl 2 (g) molecule. How does this type of bonding differ from that found in the HCl(g) molecule? How is it similar?
-
Assume you have assigned a GridPane container object to a pane reference variable. Write a code fragment that fixes the width of the containers second column at 200 pixels but lets the GridPane...
-
What were the challenges Luxola faced in attracting big brands to its platform? How did Luxola eventually manage to attract big brands? What were the primary contributing factors to Luxola's success?...
-
In the Kinetic theory of gases (you can assume an ideal gas) a typical integral that one needs to evaluate is m 3/2 Af exp(-Bv )u+ dv Af exp(-By )v dv where A=4 and 2nk T 2k T m is the mass of a...
-
Consider the two hypothetical nations of Golikia and Fretonia. Suppose they both produce only two goods, robot vacuum cleaners and catnip toys. Each country faces a trade-off when producing the two...
-
A 0.0242 m diameter coin rolls up a 12.0 inclined plane. The coin starts with an initial angular speed of 49.9 rad/s and rolls in a straight line without slipping. How much vertical height does it...
-
1. What is the discipline designator for an architectural drawing? 2. What designator is used for Survey/Mapping drawings? 3. How many numbers are allowed in a sheet name? 4. How many letters are...
-
White Limiteds most recent income statement is shown below: Required: Prepare a new contribution format income statement under each of the following conditions (consider each case independently): 1....
-
The comparative statements of financial position of Menachem NV at the beginning and end of the year 2019 appear below. Net income of ¬34,000 was reported, and dividends of ¬23,000 were paid...
-
The concentration of Pb 2+ in a solution saturated with PbBr 2 (s) is 2.14 10 -2 M. Calculate K sp for PbBr 2 .
-
Approximately 0.14 g nickel(II) hydroxide, Ni(OH) 2 (s), dissolves per liter of water at 20 C. Calculate K sp for Ni(OH) 2 (s) at this temperature.
-
Write balanced equations for the dissolution reactions and the corresponding solubility product expressions for each of the following solids. a. Ag 2 CO 3 b. Ce(IO 3 ) 3 c. BaF 2
-
INSTRUCTIONS: Using the following answer keys, you are to identify in which activity each of the transactions is classified and its effect on cash flows. Cash Flow Classification...using the capital...
-
The standard models of interest rate determination indicate risk is the main determinate of rates. Explain the types of risk that financial institution use in order to set interest rates on...
-
2 1 . ( 1 0 points ) A one - year bond has an interest rate of 6 % and is expected to fall to 3 % next year and 1 % in two years. The term premium for a two - year bond is 0 . 4 % and for a three -...

Study smarter with the SolutionInn App


