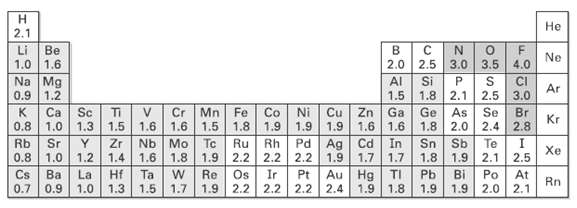
Question: Use the electro negatively table (Figure) to predict which bond in each of the following sets is more polar, and indicate the direction of bond
Use the electro negatively table (Figure) to predict which bond in each of the following sets is more polar, and indicate the direction of bond polarity for each compound.
(a) H3C ? C1 OR C1 ? C1
(b) H3C ? H OR H ? C1
(c) HO ? CH3 OR (CH3)3Si ? CH3
(d) H3C ? Li OR Li ? OH
2.1 Li Be 1.0 1.6 B. 2.0 Ne 2.5 3.0 2.5 3.0 3.5 | 4.0 Na Mg 0.9 1.2 Ca 0.8 CI Ar Al 1.5 1.8 2.1 Si Ge As Ni Cr Mn Zn Ga Se Cu Br Kr Sc Ti 1.5 1.3 1.6 1.6 Zr Nb Mo 1.2 1.4 La Hf 1.5 1.7 1.0 1.3 Fe Co 1.8 1.9 Ru Rh Tc 1.6 1.6 In 1.7 Hg| TI 1.9 1.8 1.8 2.0 2.4 2.8 1.0 Rb 1.5 1.9 1.9 Pd Ag 1.6 1.8 | 1.9 2.2 | 2.2 2.2 Sr 0.8 Cd Sb Sn 1.8 Xe Te 1.0 Cs 1.7 2.5 1.9 2.1 1.9 Ba 0.7 0.9 Bi Po 1.9 2.0 2.1 1.9 Pb At Rn Re 1.9 Pt Au Ta Os Ir 2.2 | 2.2 2.2 2.4
Step by Step Solution
3.52 Rating (176 Votes )
There are 3 Steps involved in it
a H3C CI 0 b More p... View full answer

Get step-by-step solutions from verified subject matter experts
Document Format (1 attachment)
22-C-O-A-B-R (76).docx
120 KBs Word File


