A compound consisting of yttrium(III) ions, barium(II) ions, both copper(II) and copper(III) ions, and oxide ions is
Question:
A compound consisting of yttrium(III) ions, barium(II) ions, both copper(II) and copper(III) ions, and oxide ions is a superconducting material at low temperatures. It has the formula YBa2Cu3O7−x where x is a variable between 1 and 0. To find out the value of x, you dissolve 34.02 mg of the compound in 5 mL of 1.0 M HCl. Bubbles of oxygen gas (O2) are observed as the following reaction occurs: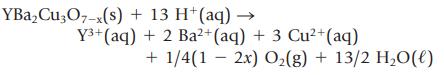
You then boil the solution, cool it, and add 10 mL of 0.70 M KI under argon. The following reaction occurs: ![]()
When this reaction is complete, a titration of the resulting solution with sodium thiosulfate requires 1.542 × 10−4 mol S2O32−(aq).
![]()
What is the value of x in YBa2Cu3O7−x?
Step by Step Answer:

Chemistry And Chemical Reactivity
ISBN: 9780357001172
10th Edition
Authors: John C. Kotz, Paul M. Treichel, John Townsend, David Treichel





