Ammonia gas can be prepared by the reaction of a metal oxide such as calcium oxide with
Question:
Ammonia gas can be prepared by the reaction of a metal oxide such as calcium oxide with ammonium chloride.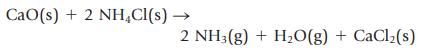
If 112 g of CaO and 224 g of NH4Cl are mixed, what is the limiting reactant, and what mass of NH3 can be produced?
Transcribed Image Text:
CaO(s) + 2 NH₂Cl(s) → 2 NH3(g) + H₂O(g) + CaCl₂(s)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 33% (3 reviews)
To determine the limiting reactant and the mass of NH3 that can be produced we should first calculat...View the full answer

Answered By

PALASH JHANWAR
I am a Chartered Accountant with AIR 45 in CA - IPCC. I am a Merit Holder ( B.Com ). The following is my educational details.
PLEASE ACCESS MY RESUME FROM THE FOLLOWING LINK: https://drive.google.com/file/d/1hYR1uch-ff6MRC_cDB07K6VqY9kQ3SFL/view?usp=sharing
3.80+
3+ Reviews
10+ Question Solved
Related Book For 

Chemistry And Chemical Reactivity
ISBN: 9780357001172
10th Edition
Authors: John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Question Posted:
Students also viewed these Sciences questions
-
What mass of silver chloride can be prepared by the reaction of 100.0 mL of 0.20 M silver nitrate with 100.0 mL of 0.15 M calcium chloride? Calculate the concentrations of each ion remaining in...
-
The following are the financial statements of Swifty Corporation. Swifty Corporation Comparative Balance Sheets December 31 Assets 2019 2018 Cash $37,200 $19,700 Accounts receivable 33,000 18,400...
-
Ammonia gas can be prepared by the following reaction: If 112 g of CaO and 224 g of NH 4 Cl are mixed, the theoretical yield of NH 3 is 68.0 g. If only 16.3 g of NH 3 is actually obtained, what is...
-
A supplier has the following financial information available: Cash: $100,000 Current Assets: $1,000,000 Fixed Assets: $1,000,000 Total Assets: $2,000,000 Current Liabilities: $500,000 Total...
-
Harrison Hotels is considering adding a spa to its current facility in order to improve its list of amenities. Operating the spa would require a fixed cost of $25,000 a year. Variable cost is...
-
Below are a balance sheet and an income statement that have been reformulated according to the templates laid out in this chapter. Ignore income taxes. Income Statement 2012 Operating revenues...
-
(See The Wide World of Fluids article titled "Smart Shocks," Section 5.3.3.) A 200-lb force applied to the end of the piston of the shock absorber shown in Fig. P5.104 causes the two ends of the...
-
Natalie Bryan, a widow who lives at 425 Flathead Way, Kalispell, Montana 59901, has three adult children (Daniel Bryan, Amanda Green, and Samantha Cruz). During the year, Natalie makes the following...
-
In the absence of air resistance, a projectile that lands at the elevation from which it was launched achieves maximum range when launched at a 45 angle. Suppose a projectile of mass m is launched...
-
The compound SF 6 is made by burning sulfur in an atmosphere of fluorine. The balanced equation is Starting with a mixture of 1.6 mol of sulfur, S 8 , and 35 mol of F 2 , (a) Which is the limiting...
-
Ethane, C 2 H 6 , burns in oxygen. (a) What are the products of the reaction? (b) Write the balanced equation for the reaction. (c) What mass of O 2 , in grams, is required for complete combustion of...
-
In 1944, W. E. Collins conveyed land to the Church of God of Prophecy. The deed said: This deed is made with the full understanding that should the property fail to be used for the Church of God, it...
-
Read the article "Needle and syringe programmes are highly cost-effective at preventing hepatitis C transmission" and answer the questions below. At what point in the research might the researchers...
-
Blossom enters into an agreement with Crane Inc. to lease a car on December 31, 2024. The following information relates to this agreement. 1. The term of the non-cancelable lease is 3 years with no...
-
If YOU were to devise a personal strategy for how to behave in challenging interpersonal situations, outline the steps to this strategy. In addition, indicate to what degree would this strategy use...
-
Last year, Harold Company produced 2,000 units and sold 1,000 units. The company had no beginning inventory. Harold incurred the following costs: Direct materials per unit Direct labor per unit $30...
-
Develop a short service quality improvement plan for the police department as a set of instructions from improving its service quality.
-
For each transaction, identify a possible source document. a. The business received $ 20,000 cash and issued common stock to stockholders. b. Purchased office supplies on account, $ 500. c. Recorded...
-
5. How much would you need to deposit in an account now in order to have $5,000 in the account in 5 years? Assume the account earns 2% interest compounded monthly. 10. You deposit $300 each month...
-
DEET is the active ingredient in many insect repellants, such as OFF TM . Starting with meta-bromotoluene and using any other reagents of your choice, devise an efficient synthesis for DEET. .Br...
-
Predict the products that are formed when diphenyl carbonate is treated with excess methyl magnesium bromide. 1) Excess MeMgBr 2) H3o*
-
When acetic acid is treated with isotopically labeled water ( 18 O, shown in red) in the presence of a catalytic amount of acid, it is observed that the isotopic label becomes incorporated at both...
-
A firm reported year-end sales of $20 million. It listed $7 million of inventory on its balance sheet. Using a 365-day year, how many days did the firm's inventory stay on the premises?
-
Apple bonds (each with a $1,000 face value and a 3.25% coupon rate) that are five years from their 10-year maturity date. how would I calculate the bond value?
-
Braverman Company has two manufacturing departments-Finishing and Fabrication. The predetermined overhead rates in Finishing and Fabrication are $26.00 per direct labor-hour and 120% of direct...

Study smarter with the SolutionInn App


